Abstract
Individuals of many species fight with conspecifics to gain access to or defend critical resources essential for survival and reproduction. Such intraspecific fighting is evolutionarily selected for in a species-, sex-, and environment-dependent manner when the value of resources secured exceeds the cost of fighting. One such example is males fighting for chances to mate with females. Recent advances in new tools open up ways to dissect the detailed neural circuit mechanisms that govern intraspecific, particularly inter-male, aggression in the model organism Mus musculus (house mouse). By targeting and functional manipulating genetically defined populations of neurons and their projections, these studies reveal a core neural circuit that controls the display of reactive male–male attacks in mice, from sensory detection to decision making and action selection. Here, we summarize these critical results. We then describe various modulatory inputs that route into the core circuit to afford state-dependent and top–down modulation of inter-male attacks. While reviewing these exciting developments, we note that how the inter-male attack circuit converges or diverges with neural circuits that mediate other forms of social interactions remain not fully understood. Finally, we emphasize the importance of combining circuit, pharmacological, and genetic analysis when studying the neural control of aggression in the future.


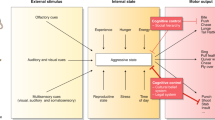
Modified from Lo et al. [94]. Brain regions that account for > 2% of the total inputs to or outputs of the VMHvlEsr1+ neurons are depicted. The size of the arrows indicates the relative connection strength, and the direction of the arrow indicates input to or output from the VMHvl. The color schemes are assigned based on the relative strength of the input and output connection from each brain region with the VMHvl. For brain regions that provide more inputs to than receive outputs from VMHvlEsr1+ neurons, if the normalized input value divided by the normalized output value (input/output) is > 4, they are colored red; and if the value is > 2 but < 4, these regions are colored orange. Similarly, for brain regions that receive more outputs from than send inputs to VMHvlEsr1+ neurons, if the normalized output value divided by the normalized input value (output/input) is > 4, they are colored dark blue; and if the value is > 2 but < 4, these regions are colored light green. All other connected brain regions with comparable input and output values are colored gray. ARC, arcuate hypothalamic nucleus; AHN, anterior hypothalamic nucleus; AVPV, anteroventral periventricular nucleus; BNST, bed nuclei of the stria terminalis; CEA, central amygdala nucleus; DMH, dorsomedial nucleus of the hypothalamus; LS, lateral septum; MeA, medial amygdala; mPOA, medial preoptic area; PA, posterior amygdala; PAG, periaqueductal gray; PB, parabrachial nucleus; PMv, ventral premammillary nucleus; PVN, paraventricular hypothalamic nucleus; PVT, paraventricular thalamus; pSI, posterior substantia innominate; SPFp, subparafascicular nucleus, parvicellular part; SUBv, subiculum, ventral part; TTd, taenia tecta, dorsal part

Similar content being viewed by others
References
Alcock J (2013) Animal behavior: an evolutionary approach, 10th edn. Sinauer Associates is an imprint of Oxford University Press, Sunderland
Tinbergen N (1989) The study of instinct. Clarendon Press, Oxford Univerisity Press, Oxford, England, New York
Territoriality and aggression learn science at scitable. https://www.nature.com/scitable/knowledge/library/territoriality-and-aggression-13240908/. Accessed 10 Mar 2021
Georgiev AV, Klimczuk ACE, Traficonte DM, Maestripieri D (2013) When violence pays: a cost-benefit analysis of aggressive behavior in animals and humans. Evol Psychol 11:678–699
Archer J (2009) Does sexual selection explain human sex differences in aggression? Behav Brain Sci 32:249–266. https://doi.org/10.1017/S0140525X09990951 (discussion 266-311)
Tobias JA, Montgomerie R, Lyon BE (2012) The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos Trans R Soc Lond B Biol Sci 367:2274–2293. https://doi.org/10.1098/rstb.2011.0280
Svare BB (1981) Maternal aggression in mammals. In: Gubernick DJ, Klopfer PH (eds) Parental care in mammals. Springer, US, Boston, MA, pp 179–210
Takahashi A, Miczek KA (2014) Neurogenetics of aggressive behavior: studies in Rodents. In: Miczek KA, Meyer-Lindenberg A (eds) Neuroscience of aggression. Springer, Berlin, Heidelberg, pp 3–44
Koolhaas JM, Coppens CM, de Boer SF et al (2013) The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. https://doi.org/10.3791/4367
Panksepp J (1971) Aggression elicited by electrical stimulation of the hypothalamus in albino rats. Physiol Behav 6:321–329. https://doi.org/10.1016/0031-9384(71)90163-6
Kruk MR, van der Poel AM, de Vos-Frerichs TP (1979) The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour 70:292–322. https://doi.org/10.1163/156853979x00106
Siegel A, Roeling TA, Gregg TR, Kruk MR (1999) Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev 23:359–389. https://doi.org/10.1016/s0149-7634(98)00040-2
Popova NK, Kulikov AV (1986) Genetic analysis of “Spontaneous” intermale aggression in mice. Aggressive Behav 12:425–431. https://doi.org/10.1002/1098-2337(1986)12:6%3c425::AID-AB2480120605%3e3.0.CO;2-A
Zhang-James Y, Fernàndez-Castillo N, Hess JL et al (2019) An integrated analysis of genes and functional pathways for aggression in human and rodent models. Mol Psychiatry 24:1655–1667. https://doi.org/10.1038/s41380-018-0068-7
Leroy F, Park J, Asok A et al (2018) A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 564:213–218. https://doi.org/10.1038/s41586-018-0772-0
Chen A-X, Yan J-J, Zhang W et al (2020) Specific hypothalamic neurons required for sensing conspecific male cues relevant to inter-male aggression. Neuron 108:763-774.e6. https://doi.org/10.1016/j.neuron.2020.08.025
Remedios R, Kennedy A, Zelikowsky M et al (2017) Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550:388–392. https://doi.org/10.1038/nature23885
Lee H, Kim D-W, Remedios R et al (2014) Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509:627–632. https://doi.org/10.1038/nature13169
Chang C-H, Gean P-W (2019) The ventral hippocampus controls stress-provoked impulsive aggression through the ventromedial hypothalamus in post-weaning social isolation mice. Cell Rep 28:1195-1205.e3. https://doi.org/10.1016/j.celrep.2019.07.005
Stagkourakis S, Spigolon G, Williams P et al (2018) A neural network for intermale aggression to establish social hierarchy. Nat Neurosci 21:834–842. https://doi.org/10.1038/s41593-018-0153-x
Takahashi A, Nagayasu K, Nishitani N et al (2014) Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS One 9:e94657. https://doi.org/10.1371/journal.pone.0094657
van Heukelum S, Tulva K, Geers FE et al (2021) A central role for anterior cingulate cortex in the control of pathological aggression. Curr Biol 31:2321-2333.e5. https://doi.org/10.1016/j.cub.2021.03.062
Atasoy D, Sternson SM (2018) Chemogenetic tools for causal cellular and neuronal biology. Physiol Rev 98:391–418. https://doi.org/10.1152/physrev.00009.2017
Zha X, Xu X (2015) Dissecting the hypothalamic pathways that underlie innate behaviors. Neurosci Bull 31:629–648. https://doi.org/10.1007/s12264-015-1564-2
Luo L, Callaway EM, Svoboda K (2018) Genetic dissection of neural circuits: a decade of progress. Neuron 98:256–281. https://doi.org/10.1016/j.neuron.2018.03.040
Kim CK, Adhikari A, Deisseroth K (2017) Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 18:222–235. https://doi.org/10.1038/nrn.2017.15
Callaway EM, Luo L (2015) Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci 35:8979–8985. https://doi.org/10.1523/JNEUROSCI.0409-15.2015
Adamantidis A, Arber S, Bains JS et al (2015) Optogenetics: 10 years after ChR2 in neurons–views from the community. Nat Neurosci 18:1202–1212. https://doi.org/10.1038/nn.4106
Rajasethupathy P, Ferenczi E, Deisseroth K (2016) Targeting neural circuits. Cell 165:524–534. https://doi.org/10.1016/j.cell.2016.03.047
Xu X, Holmes TC, Luo M-H et al (2020) Viral vectors for neural circuit mapping and recent advances in trans-synaptic anterograde tracers. Neuron 107:1029–1047. https://doi.org/10.1016/j.neuron.2020.07.010
Yamaguchi T, Lin D (2018) Functions of medial hypothalamic and mesolimbic dopamine circuitries in aggression. Curr Opin Behav Sci 24:104–112. https://doi.org/10.1016/j.cobeha.2018.06.011
Wei D, Talwar V, Lin D (2021) Neural circuits of social behaviors: innate yet flexible. Neuron. https://doi.org/10.1016/j.neuron.2021.02.012
Falkner AL, Lin D (2014) Recent advances in understanding the role of the hypothalamic circuit during aggression. Front Syst Neurosci 8:168. https://doi.org/10.3389/fnsys.2014.00168
Hashikawa K, Hashikawa Y, Falkner A, Lin D (2016) The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol 38:27–37. https://doi.org/10.1016/j.conb.2016.01.006
Hashikawa K, Hashikawa Y, Lischinsky J, Lin D (2018) The neural mechanisms of sexually dimorphic aggressive behaviors. Trends Genet 34:755–776. https://doi.org/10.1016/j.tig.2018.07.001
Hashikawa Y, Hashikawa K, Falkner AL, Lin D (2017) Ventromedial hypothalamus and the generation of aggression. Front Syst Neurosci 11:94. https://doi.org/10.3389/fnsys.2017.00094
Chen P, Hong W (2018) Neural circuit mechanisms of social behavior. Neuron 98:16–30. https://doi.org/10.1016/j.neuron.2018.02.026
Anderson DJ (2016) Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci 17:692–704. https://doi.org/10.1038/nrn.2016.125
Anderson DJ (2012) Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol Psychiatry 71:1081–1089. https://doi.org/10.1016/j.biopsych.2011.11.012
Flanigan M, Aleyasin H, Takahashi A et al (2017) An emerging role for the lateral habenula in aggressive behavior. Pharmacol Biochem Behav 162:79–86. https://doi.org/10.1016/j.pbb.2017.05.003
Golden SA, Jin M, Shaham Y (2019) Animal models of (or for) aggression reward, addiction, and relapse: behavior and circuits. J Neurosci 39:3996–4008. https://doi.org/10.1523/JNEUROSCI.0151-19.2019
Goodwin NL, Nilsson SRO, Golden SA (2020) Rage against the machine: advancing the study of aggression ethology via machine learning. Psychopharmacology 237:2569–2588. https://doi.org/10.1007/s00213-020-05577-x
Aleyasin H, Flanigan ME, Russo SJ (2018) Neurocircuitry of aggression and aggression seeking behavior: nose poking into brain circuitry controlling aggression. Curr Opin Neurobiol 49:184–191. https://doi.org/10.1016/j.conb.2018.02.013
Wu MV, Shah NM (2011) Control of masculinization of the brain and behavior. Curr Opin Neurobiol 21:116–123. https://doi.org/10.1016/j.conb.2010.09.014
Bayless DW, Shah NM (2016) Genetic dissection of neural circuits underlying sexually dimorphic social behaviours. Philos Trans R Soc Lond B Biol Sci 371:20150109. https://doi.org/10.1098/rstb.2015.0109
Yang T, Shah NM (2016) Molecular and neural control of sexually dimorphic social behaviors. Curr Opin Neurobiol 38:89–95. https://doi.org/10.1016/j.conb.2016.04.015
Manoli DS, Fan P, Fraser EJ, Shah NM (2013) Neural control of sexually dimorphic behaviors. Curr Opin Neurobiol 23:330–338. https://doi.org/10.1016/j.conb.2013.04.005
McKinsey G, Ahmed OM, Shah NM (2018) Neural control of sexually dimorphic social behaviors. Curr Opin Physiol 6:89–95. https://doi.org/10.1016/j.cophys.2018.08.003
Yang CF, Shah NM (2014) Representing sex in the brain, one module at a time. Neuron 82:261–278. https://doi.org/10.1016/j.neuron.2014.03.029
de Boer SF, Olivier B, Veening J, Koolhaas JM (2015) The neurobiology of offensive aggression: revealing a modular view. Physiol Behav 146:111–127. https://doi.org/10.1016/j.physbeh.2015.04.040
Lischinsky JE, Lin D (2020) Neural mechanisms of aggression across species. Nat Neurosci 23:1317–1328. https://doi.org/10.1038/s41593-020-00715-2
Hurst JL, Payne CE, Nevison CM et al (2001) Individual recognition in mice mediated by major urinary proteins. Nature 414:631–634. https://doi.org/10.1038/414631a
Stowers L, Kuo T-H (2015) Mammalian pheromones: emerging properties and mechanisms of detection. Curr Opin Neurobiol 34:103–109. https://doi.org/10.1016/j.conb.2015.02.005
Stowers L, Marton TF (2005) What is a pheromone? Mammalian pheromones reconsidered. Neuron 46:699–702. https://doi.org/10.1016/j.neuron.2005.04.032
Spehr M, Spehr J, Ukhanov K et al (2006) Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell Mol Life Sci 63:1476–1484. https://doi.org/10.1007/s00018-006-6109-4
Armstrong SD, Robertson DHL, Cheetham SA et al (2005) Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone. Biochem J 391:343–350. https://doi.org/10.1042/BJ20050404
Kimoto H, Haga S, Sato K, Touhara K (2005) Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature 437:898–901. https://doi.org/10.1038/nature04033
Zhang J-X, Rao X-P, Sun L et al (2007) Putative chemical signals about sex, individuality, and genetic background in the preputial gland and urine of the house mouse (Mus musculus). Chem Senses 32:293–303. https://doi.org/10.1093/chemse/bjl058
Lin DY, Zhang S-Z, Block E, Katz LC (2005) Encoding social signals in the mouse main olfactory bulb. Nature 434:470–477. https://doi.org/10.1038/nature03414
Chamero P, Marton TF, Logan DW et al (2007) Identification of protein pheromones that promote aggressive behaviour. Nature 450:899–902. https://doi.org/10.1038/nature05997
Kaur AW, Ackels T, Kuo T-H et al (2014) Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell 157:676–688. https://doi.org/10.1016/j.cell.2014.02.025
Hattori T, Osakada T, Matsumoto A et al (2016) Self-exposure to the male pheromone ESP1 enhances male aggressiveness in mice. Curr Biol 26:1229–1234. https://doi.org/10.1016/j.cub.2016.03.029
Liu Q, Zhang Y, Wang P et al (2019) Two preputial gland-secreted pheromones evoke sexually dimorphic neural pathways in the mouse vomeronasal system. Front Cell Neurosci. https://doi.org/10.3389/fncel.2019.00455
Leypold BG, Yu CR, Leinders-Zufall T et al (2002) Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA 99:6376–6381. https://doi.org/10.1073/pnas.082127599
Stowers L, Holy TE, Meister M et al (2002) Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295:1493–1500. https://doi.org/10.1126/science.1069259
Chamero P, Katsoulidou V, Hendrix P et al (2011) G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci USA 108:12898–12903. https://doi.org/10.1073/pnas.1107770108
Norlin EM, Gussing F, Berghard A (2003) Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr Biol 13:1214–1219. https://doi.org/10.1016/s0960-9822(03)00452-4
Fraser EJ, Shah NM (2014) Complex chemosensory control of female reproductive behaviors. PLoS ONE 9:e90368. https://doi.org/10.1371/journal.pone.0090368
Mandiyan VS, Coats JK, Shah NM (2005) Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci 8:1660–1662. https://doi.org/10.1038/nn1589
Choi GB, Dong H-W, Murphy AJ et al (2005) Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46:647–660. https://doi.org/10.1016/j.neuron.2005.04.011
He J, Ma L, Kim S et al (2008) Encoding gender and individual information in the mouse vomeronasal organ. Science 320:535–538. https://doi.org/10.1126/science.1154476
Meeks JP, Arnson HA, Holy TE (2010) Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci 13:723–730. https://doi.org/10.1038/nn.2546
Luo M, Fee MS, Katz LC (2003) Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science 299:1196–1201. https://doi.org/10.1126/science.1082133
Li Y, Mathis A, Grewe BF et al (2017) Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171:1176-1190.e17. https://doi.org/10.1016/j.cell.2017.10.015
Ben-Shaul Y, Katz LC, Mooney R, Dulac C (2010) In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci USA 107:5172–5177. https://doi.org/10.1073/pnas.0915147107
Bergan JF, Ben-Shaul Y, Dulac C (2014) Sex-specific processing of social cues in the medial amygdala. Elife 3:e02743. https://doi.org/10.7554/eLife.02743
Yao S, Bergan J, Lanjuin A, Dulac C (2017) Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife. https://doi.org/10.7554/eLife.31373
Hong W, Kim D-W, Anderson DJ (2014) Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158:1348–1361. https://doi.org/10.1016/j.cell.2014.07.049
Baleisyte A, Schneggenburger R, Kochubey O (2021) Optogenetic stimulation of medial amygdala GABA neurons with kinetically different channelrhodopsin variants yield opposite behavioral outcomes. bioRxiv. https://doi.org/10.1101/2021.06.30.450543
Chen PB, Hu RK, Wu YE et al (2019) Sexually dimorphic control of parenting behavior by the medial amygdala. Cell 176:1206-1221.e18. https://doi.org/10.1016/j.cell.2019.01.024
Unger EK, Burke KJ, Yang CF et al (2015) Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10:453–462. https://doi.org/10.1016/j.celrep.2014.12.040
von Campenhausen H, Mori K (2000) Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur J Neurosci 12:33–46. https://doi.org/10.1046/j.1460-9568.2000.00879.x
Gungor NZ, Paré D (2016) Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci 36:8038–8049. https://doi.org/10.1523/JNEUROSCI.0856-16.2016
Lebow MA, Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 21:450–463. https://doi.org/10.1038/mp.2016.1
Miller SM, Marcotulli D, Shen A, Zweifel LS (2019) Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat Neurosci 22:565–575. https://doi.org/10.1038/s41593-019-0337-z
Dong HW, Petrovich GD, Swanson LW (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38:192–246. https://doi.org/10.1016/s0165-0173(01)00079-0
Bayless DW, Yang T, Mason MM et al (2019) Limbic neurons shape sex recognition and social behavior in sexually naive males. Cell 176:1190-1205.e20. https://doi.org/10.1016/j.cell.2018.12.041
Newman SW (1999) The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 877:242–257. https://doi.org/10.1111/j.1749-6632.1999.tb09271.x
Wei Y-C, Wang S-R, Jiao Z-L et al (2018) Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun 9:279. https://doi.org/10.1038/s41467-017-02648-0
Gao S-C, Wei Y-C, Wang S-R, Xu X-H (2019) Medial preoptic area modulates courtship ultrasonic vocalization in adult male mice. Neurosci Bull 35:697–708. https://doi.org/10.1007/s12264-019-00365-w
Kohl J, Babayan BM, Rubinstein ND et al (2018) Functional circuit architecture underlying parental behaviour. Nature 556:326–331. https://doi.org/10.1038/s41586-018-0027-0
Wu Z, Autry AE, Bergan JF et al (2014) Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509:325–330. https://doi.org/10.1038/nature13307
Fang Y-Y, Yamaguchi T, Song SC et al (2018) A hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron 98:192-207.e10. https://doi.org/10.1016/j.neuron.2018.02.019
Karigo T, Kennedy A, Yang B et al (2021) Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature 589:258–263. https://doi.org/10.1038/s41586-020-2995-0
Lo L, Yao S, Kim D-W et al (2019) Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proc Natl Acad Sci U S A 116:7503–7512. https://doi.org/10.1073/pnas.1817503116
Soden ME, Miller SM, Burgeno LM et al (2016) Genetic isolation of hypothalamic neurons that regulate context-specific male social behavior. Cell Rep 16:304–313. https://doi.org/10.1016/j.celrep.2016.05.067
Falkner AL, Dollar P, Perona P et al (2014) Decoding ventromedial hypothalamic neural activity during male mouse aggression. J Neurosci 34:5971–5984. https://doi.org/10.1523/JNEUROSCI.5109-13.2014
Lin D, Boyle MP, Dollar P et al (2011) Functional identification of an aggression locus in the mouse hypothalamus. Nature 470:221–226. https://doi.org/10.1038/nature09736
Yang T, Yang CF, Chizari MD et al (2017) Social control of hypothalamus-mediated male aggression. Neuron 95:955-970.e4. https://doi.org/10.1016/j.neuron.2017.06.046
Yang CF, Chiang MC, Gray DC et al (2013) Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153:896–909. https://doi.org/10.1016/j.cell.2013.04.017
Hashikawa K, Hashikawa Y, Tremblay R et al (2017) Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat Neurosci 20:1580–1590. https://doi.org/10.1038/nn.4644
Kruk MR (1991) Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev 15:527–538. https://doi.org/10.1016/s0149-7634(05)80144-7
Wang L, Talwar V, Osakada T et al (2019) Hypothalamic control of conspecific self-defense. Cell Rep 26:1747-1758.e5. https://doi.org/10.1016/j.celrep.2019.01.078
Falkner AL, Grosenick L, Davidson TJ et al (2016) Hypothalamic control of male aggression-seeking behavior. Nat Neurosci 19:596–604. https://doi.org/10.1038/nn.4264
Bandler R, Keay KA (1996) Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res 107:285–300. https://doi.org/10.1016/s0079-6123(08)61871-3
Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17:379–389. https://doi.org/10.1016/0166-2236(94)90047-7
Falkner AL, Wei D, Song A et al (2020) Hierarchical representations of aggression in a hypothalamic-midbrain circuit. Neuron 106:637-648.e6. https://doi.org/10.1016/j.neuron.2020.02.014
Tovote P, Esposito MS, Botta P et al (2016) Midbrain circuits for defensive behaviour. Nature 534:206–212. https://doi.org/10.1038/nature17996
Zhu Z, Ma Q, Miao L et al (2021) A substantia innominata-midbrain circuit controls a general aggressive response. Neuron 109:1540-1553.e9. https://doi.org/10.1016/j.neuron.2021.03.002
Todd WD, Fenselau H, Wang JL et al (2018) A hypothalamic circuit for the circadian control of aggression. Nat Neurosci 21:717–724. https://doi.org/10.1038/s41593-018-0126-0
Saper CB, Lu J, Chou TC, Gooley J (2005) The hypothalamic integrator for circadian rhythms. Trends Neurosci 28:152–157. https://doi.org/10.1016/j.tins.2004.12.009
Padilla SL, Qiu J, Soden ME et al (2016) Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci 19:734–741. https://doi.org/10.1038/nn.4274
Sternson SM, Eiselt A-K (2017) Three pillars for the neural control of appetite. Annu Rev Physiol 79:401–423. https://doi.org/10.1146/annurev-physiol-021115-104948
Zelikowsky M, Hui M, Karigo T et al (2018) The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173:1265-1279.e19. https://doi.org/10.1016/j.cell.2018.03.037
Cádiz-Moretti B, Otero-García M, Martínez-García F, Lanuza E (2016) Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct Funct 221:1033–1065. https://doi.org/10.1007/s00429-014-0954-y
Pardo-Bellver C, Cadiz-Moretti B, Novejarque A et al (2012) Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat. https://doi.org/10.3389/fnana.2012.00033
Puglisi-Allegra S, Renzi P (1977) A technique for the measurement of aggressive behavior in mice. Behav Res Meth Instru 9:503–504. https://doi.org/10.3758/BF03213985
Nordman JC, Ma X, Gu Q et al (2020) Potentiation of divergent medial amygdala pathways drives experience-dependent aggression escalation. J Neurosci 40:4858–4880. https://doi.org/10.1523/JNEUROSCI.0370-20.2020
Nordman J, Ma X, Li Z (2020) Traumatic stress induces prolonged aggression increase through synaptic potentiation in the medial amygdala circuits. eNeuro. https://doi.org/10.1523/ENEURO.0147-20.2020
Wong LC, Wang L, D’Amour JA et al (2016) Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Curr Biol 26:593–604. https://doi.org/10.1016/j.cub.2015.12.065
Oliva A, Fernández-Ruiz A, Leroy F, Siegelbaum SA (2020) Hippocampal CA2 sharp-wave ripples reactivate and promote social memory. Nature 587:264–269. https://doi.org/10.1038/s41586-020-2758-y
Hitti FL, Siegelbaum SA (2014) The hippocampal CA2 region is essential for social memory. Nature 508:88–92. https://doi.org/10.1038/nature13028
Bendesky A, Kwon Y-M, Lassance J-M et al (2017) The genetic basis of parental care evolution in monogamous mice. Nature 544:434–439. https://doi.org/10.1038/nature22074
Gabor CS, Phan A, Clipperton-Allen AE et al (2012) Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci 126:97–109. https://doi.org/10.1037/a0026464
Fremeau RT, Kam K, Qureshi T et al (2004) Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 304:1815–1819. https://doi.org/10.1126/science.1097468
Stagkourakis S, Spigolon G, Liu G, Anderson DJ (2020) Experience-dependent plasticity in an innate social behavior is mediated by hypothalamic LTP. Proc Natl Acad Sci USA 117:25789–25799. https://doi.org/10.1073/pnas.2011782117
Zha X, Wang L, Jiao Z-L et al (2020) VMHvl-projecting Vglut1+ neurons in the posterior amygdala gate territorial aggression. Cell Rep 31:107517. https://doi.org/10.1016/j.celrep.2020.03.081
Yamaguchi T, Wei D, Song SC et al (2020) Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat Neurosci 23:1111–1124. https://doi.org/10.1038/s41593-020-0675-x
Siever LJ (2008) Neurobiology of aggression and violence. AJP 165:429–442. https://doi.org/10.1176/appi.ajp.2008.07111774
Carlén M (2017) What constitutes the prefrontal cortex? Science 358:478–482. https://doi.org/10.1126/science.aan8868
Siegel A, Edinger H, Lowenthal H (1974) Effects of electrical stimulation of the medial aspect of the prefrontal cortex upon attack behavior in cats. Brain Res 66:467–479. https://doi.org/10.1016/0006-8993(74)90061-4
Giancola PR (1995) EVidence for dorsolateral and orbital prefrontal cortical involvement in the expression of aggressive behavior. Aggressive Behav 21:431–450. https://doi.org/10.1002/1098-2337(1995)21:6%3c431::AID-AB2480210604%3e3.0.CO;2-Q
Halász J, Tóth M, Kalló I et al (2006) The activation of prefrontal cortical neurons in aggression—a double labeling study. Behav Brain Res 175:166–175. https://doi.org/10.1016/j.bbr.2006.08.019
Biro L, Sipos E, Bruzsik B et al (2018) Task division within the prefrontal cortex: distinct neuron populations selectively control different aspects of aggressive behavior via the hypothalamus. J Neurosci 38:4065–4075. https://doi.org/10.1523/JNEUROSCI.3234-17.2018
van Heukelum S, Tulva K, Geers FE et al (2021) A central role for anterior cingulate cortex in the control of pathological aggression. Curr Biol 31:2321-2333.e5. https://doi.org/10.1016/j.cub.2021.03.062
Franklin TB, Silva BA, Perova Z et al (2017) Prefrontal cortical control of a brainstem social behavior circuit. Nat Neurosci 20:260–270. https://doi.org/10.1038/nn.4470
Edwards DA (1968) Mice: fighting by neonatally androgenized females. Science 161:1027–1028. https://doi.org/10.1126/science.161.3845.1027
Edwards DA (1971) Neonatal administration of androstenedione, testosterone or testosterone propionate: effects on ovulation, sexual receptivity and aggressive behavior in female mice. Physiol Behav 6:223–228. https://doi.org/10.1016/0031-9384(71)90030-8
Barkley MS, Goldman BD (1977) Testosterone-induced aggression in adult female mice. Horm Behav 9:76–84. https://doi.org/10.1016/0018-506x(77)90052-6
Peters PJ, Bronson FH, Whitsett JM (1972) Neonatal castration and intermale aggression in mice. Physiol Behav 8:265–268. https://doi.org/10.1016/0031-9384(72)90371-X
Juntti SA, Tollkuhn J, Wu MV et al (2010) The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66:260–272. https://doi.org/10.1016/j.neuron.2010.03.024
Soden ME, Miller SM, Burgeno LM et al (2016) Genetic isolation of hypothalamic neurons that regulate context-specific male social behavior. Cell Rep 16:304–313. https://doi.org/10.1016/j.celrep.2016.05.067
Siegel A, Bhatt S, Bhatt R, Zalcman SS (2007) The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr Neuropharmacol 5:135–147. https://doi.org/10.2174/157015907780866929
Saudou F, Amara DA, Dierich A et al (1994) Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265:1875–1878. https://doi.org/10.1126/science.8091214
Fish EW, DeBold JF, Miczek KA (2005) Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology 182:116–127. https://doi.org/10.1007/s00213-005-0064-x
Yu Q, Teixeira CM, Mahadevia D et al (2014) Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry 19:688–698. https://doi.org/10.1038/mp.2014.10
Narvaes R (2014) Aggressive behavior and three neurotransmitters: dopamine, GABA, and serotonin—a review of the last 10 years. Psychol Neurosci 7:601. https://doi.org/10.3922/j.psns.2014.4.20
de Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA (2005) Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 526:51–64. https://doi.org/10.1016/j.ejphar.2005.10.004
Miczek KA, Fish EW, De Bold JF, De Almeida RMM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology 163:434–458. https://doi.org/10.1007/s00213-002-1139-6
Miczek KA, Maxson SC, Fish EW, Faccidomo S (2001) Aggressive behavioral phenotypes in mice. Behav Brain Res 125:167–181. https://doi.org/10.1016/s0166-4328(01)00298-4
Tecott LH, Barondes SH (1996) Genes and aggressiveness. Behavioral genetics. Curr Biol 6:238–240. https://doi.org/10.1016/s0960-9822(02)00466-9
Hendricks TJ, Fyodorov DV, Wegman LJ et al (2003) Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37:233–247. https://doi.org/10.1016/s0896-6273(02)01167-4
Olivier B (2004) Serotonin and aggression. Ann NY Acad Sci 1036:382–392. https://doi.org/10.1196/annals.1330.022
Nelson RJ, Trainor BC (2007) Neural mechanisms of aggression. Nat Rev Neurosci 8:536–546. https://doi.org/10.1038/nrn2174
Chiavegatto S, Dawson VL, Mamounas LA et al (2001) Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 98:1277–1281. https://doi.org/10.1073/pnas.031487198
Ren J, Friedmann D, Xiong J et al (2018) Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175:472-487.e20. https://doi.org/10.1016/j.cell.2018.07.043
Weissbourd B, Ren J, DeLoach KE et al (2014) Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83:645–662. https://doi.org/10.1016/j.neuron.2014.06.024
Ren J, Isakova A, Friedmann D et al (2019) Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife. https://doi.org/10.7554/eLife.49424
Nautiyal KM, Tanaka KF, Barr MM et al (2015) Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86:813–826. https://doi.org/10.1016/j.neuron.2015.03.041
Wan J, Peng W, Li X et al (2021) A genetically encoded sensor for measuring serotonin dynamics. Nat Neurosci. https://doi.org/10.1038/s41593-021-00823-7
Unger EK, Keller JP, Altermatt M et al (2020) Directed evolution of a selective and sensitive serotonin sensor via machine learning. Cell 183:1986-2002.e26. https://doi.org/10.1016/j.cell.2020.11.040
Stork O, Ji FY, Kaneko K et al (2000) Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res 865:45–58. https://doi.org/10.1016/s0006-8993(00)02206-x
Liu G-X, Liu S, Cai G-Q et al (2007) Reduced aggression in mice lacking GABA transporter subtype 1. J Neurosci Res 85:649–655. https://doi.org/10.1002/jnr.21148
Clement J, Simler S, Ciesielski L et al (1987) Age-dependent changes of brain GABA levels, turnover rates and shock-induced aggressive behavior in inbred strains of mice. Pharmacol Biochem Behav 26:83–88. https://doi.org/10.1016/0091-3057(87)90538-7
Lee G, Gammie SC (2009) GABAA receptor signaling in the lateral septum regulates maternal aggression in mice. Behav Neurosci 123:1169–1177. https://doi.org/10.1037/a0017535
Nikulina ÉM, Kapralova NS (1992) Role of dopamine receptors in the regulation of aggression in mice; relationship to genotype. Neurosci Behav Physiol 22:364–369. https://doi.org/10.1007/BF01186627
Vukhac KL, Sankoorikal EB, Wang Y (2001) Dopamine D2L receptor- and age-related reduction in offensive aggression. NeuroReport 12:1035–1038. https://doi.org/10.1097/00001756-200104170-00034
Lewis MH, Gariépy JL, Gendreau P et al (1994) Social reactivity and D1 dopamine receptors: studies in mice selectively bred for high and low levels of aggression. Neuropsychopharmacology 10:115–122. https://doi.org/10.1038/npp.1994.13
Niederkofler V, Asher TE, Okaty BW et al (2016) Identification of serotonergic neuronal modules that affect aggressive behavior. Cell Rep 17:1934–1949. https://doi.org/10.1016/j.celrep.2016.10.063
Bortolato M, Floris G, Shih JC (2018) From aggression to autism: new perspectives on the behavioral sequelae of monoamine oxidase deficiency. J Neural Transm (Vienna) 125:1589–1599. https://doi.org/10.1007/s00702-018-1888-y
Kolla NJ, Bortolato M (2020) The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: a tale of mice and men. Prog Neurobiol 194:101875. https://doi.org/10.1016/j.pneurobio.2020.101875
Jun JJ, Steinmetz NA, Siegle JH et al (2017) Fully integrated silicon probes for high-density recording of neural activity. Nature 551:232–236. https://doi.org/10.1038/nature24636
Sych Y, Chernysheva M, Sumanovski LT, Helmchen F (2019) High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat Methods 16:553–560. https://doi.org/10.1038/s41592-019-0400-4
Siegel M, Buschman TJ, Miller EK (2015) Cortical information flow during flexible sensorimotor decisions. Science 348:1352–1355. https://doi.org/10.1126/science.aab0551
Mathis A, Schneider S, Lauer J, Mathis MW (2020) A primer on motion capture with deep learning: principles, pitfalls, and perspectives. Neuron 108(1):44-65. https://doi.org/10.1016/j.neuron.2020.09.017
Nilsson SR, Goodwin NL, Choong JJ et al (2020) Simple behavioral analysis (SimBA)—an open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv. https://doi.org/10.1101/2020.04.19.049452
Zeng H, Sanes JR (2017) Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci 18:530–546. https://doi.org/10.1038/nrn.2017.85
Pfaff D, Choleris E, Ogawa S (2005) Genes for sex hormone receptors controlling mouse aggression. Novartis Found Symp 268:78–89 (discussion 89-95, 96–99)
Sano K, Nakata M, Musatov S et al (2016) Pubertal activation of estrogen receptor α in the medial amygdala is essential for the full expression of male social behavior in mice. Proc Natl Acad Sci USA 113:7632–7637. https://doi.org/10.1073/pnas.1524907113
Ogawa S, Lubahn DB, Korach KS, Pfaff DW (1996) Aggressive behaviors of transgenic estrogen-receptor knockout male mice. Ann NY Acad Sci 794:384–385. https://doi.org/10.1111/j.1749-6632.1996.tb32549.x
Ogawa S, Tsukahara S, Choleris E, Vasudevan N (2020) Estrogenic regulation of social behavior and sexually dimorphic brain formation. Neurosci Biobehav Rev 110:46–59. https://doi.org/10.1016/j.neubiorev.2018.10.012
Acknowledgements
We thank members of the Xu lab for their comments on the manuscript. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grantXDB32000000), the Shanghai Municipal Science and Technology Major Project (grant no. 2018SHZDZX05), the National Nature Science Foundation of China (grant nos. 31871066 and 31922028), China Postdoctoral Science Foundation (Grant No. 2020TQ0333 and 2020M681416).
Author information
Authors and Affiliations
Contributions
XZ and X-HX made the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zha, X., Xu, XH. Neural circuit mechanisms that govern inter-male attack in mice. Cell. Mol. Life Sci. 78, 7289–7307 (2021). https://doi.org/10.1007/s00018-021-03956-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03956-x