Abstract
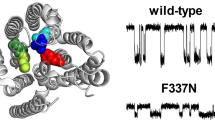
Positively charged amino acid side-chains play important roles in anion binding and permeation through the CFTR chloride channel. One pore-lining lysine residue in particular (K95) has been shown to be indispensable for anion binding, conductance, and selectivity. Here, we use functional investigation of CFTR to show that a nearby arginine (R134) plays a functionally analogous role. Removal of this positive charge (in the R134Q mutant) drastically reduces single-channel conductance, weakens binding of both permeant and blocking anions, and abolishes the normal anion conductance selectivity pattern. Each of these functional effects was reversed by a second-site mutation (S1141K) that introduces an ectopic positive charge to a nearby pore-lining residue. Substituted cysteine accessibility experiments confirm that R134—but not nearby residues in the same transmembrane helix—is accessible within the pore lumen. These results suggest that K95 and R134, which are very close together within the inner vestibule of the pore, play analogous, important roles, and that both are required for the normal anion binding and anion conductance properties of the pore. Nevertheless, that fact that both positive charges can be “transplanted” to other sites in the inner vestibule with little effect on channel permeation properties indicates that it is the overall number of charges—rather than their exact locations—that controls pore function.







Similar content being viewed by others
Availability of data and material
All data generated or analysed during this study are included in this published article. The materials used in this study are available from the corresponding author, upon reasonable request.
Abbreviations
- BHK:
-
Baby hamster kidney
- CF:
-
Cystic fibrosis
- CFTR:
-
CF transmembrane conductance regulator
- MD:
-
Molecular dynamics
- MSD:
-
Membrane-spanning domain
- MTSES:
-
[2-Sulfonatoethyl] methanethiosulfonate
- MTSET:
-
[2-(Trimethylammonium)ethyl] methanethiosulfonate
- NBD:
-
Nucleotide binding domain
- NPPB:
-
5-Nitro-2-(3-phenylpropylamino)benzoic acid
- TES:
-
N-Tris[hydroxymethyl]methyl-2-aminoethanesulfonate
- TM:
-
Transmembrane helix
- TME:
-
Transmembrane helix extension
References
Castellani C, Assael BM (2017) Cystic fibrosis: a clinical view. Cell Mol Life Sci 74:129–140
Liu F, Zhang Z, Csanády L, Gadsby DC, Chen J (2017) Molecular structure of the human CFTR ion channel. Cell 169:85–95
Zhang Z, Liu F, Chen J (2018) Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc Natl Acad Sci USA 115:12757–12762
Linsdell P (2017) Architecture and functional properties of the CFTR channel pore. Cell Mol Life Sci 74:67–83
Hwang T-C, Yeh J-T, Zhang J, Yu Y-C, Yeh H-I, Destefano S (2018) Structural mechanisms of CFTR function and dysfunction. J Gen Physiol 150:539–570
Csanády L, Vergani P, Gadsby DC (2019) Structure, gating, and regulation of the CFTR anion channel. Physiol Rev 99:707–738
Smith SS, Liu X, Zhang Z-R, Sun F, Kriewall TE, McCarty NA, Dawson DC (2001) CFTR: covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J Gen Physiol 118:407–431
Gong X, Linsdell P (2003) Molecular determinants and role of an anion binding site in the external mouth of the CFTR chloride channel pore. J Physiol 549:387–397
Zhou J-J, Fatehi M, Linsdell P (2008) Identification of positive charges situated at the outer mouth of the CFTR chloride channel pore. Pflügers Arch 457:351–360
St. Aubin CN, Linsdell P, (2006) Positive charges at the intracellular mouth of the pore regulate anion conduction in the CFTR chloride channel. J Gen Physiol 128:535–545
El Hiani Y, Linsdell P (2015) Functional architecture of the cytoplasmic entrance to the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 290:15855–15865
Li M-S, Cowley EA, El Hiani Y, Linsdell P (2018) Functional organization of cytoplasmic portals controlling access to the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel pore. J Biol Chem 293:5649–5658
Beck EJ, Yang Y, Yaemsiri S, Raghuram V (2008) Conformational changes in a pore-lining helix coupled to cystic fibrosis transmembrane conductance regulator channel gating. J Biol Chem 283:4957–4966
Wang W, El Hiani Y, Linsdell P (2011) Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Gen Physiol 138:165–178
Gao X, Bai Y, Hwang T-C (2013) Cysteine scanning of CFTR’s first transmembrane segment reveals its plausible roles in gating and permeation. Biophys J 104:786–797
Linsdell P (2014) Cystic fibrosis transmembrane conductance regulator chloride channel blockers: pharmacological, biophysical and physiological relevance. World J Biol Chem 5:26–39
Linsdell P (2021) On the relationship between anion binding and chloride conductance in the CFTR anion channel. Biochim Biophys Acta 1863:183558
Linsdell P (2005) Location of a common inhibitor binding site in the cytoplasmic vestibule of the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 280:8945–8950
Zhou J-J, Li M-S, Qi J, Linsdell P (2010) Regulation of conductance by the number of fixed positive charges in the intracellular vestibule of the CFTR chloride channel pore. J Gen Physiol 135:229–245
Ge N, Muise CN, Gong X, Linsdell P (2004) Direct comparison of the functional roles played by different transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 279:55283–55289
Linsdell P (2016) Anion conductance selectivity mechanism of the CFTR chloride channel. Biochim Biophys Acta 1858:740–747
El Hiani P, Linsdell P (2012) Tuning of CFTR chloride channel function by location of positive charges within the pore. Biophys J 103:1719–1726
Linsdell P (2015) Interactions between permeant and blocking anions inside the CFTR chloride channel pore. Biochim Biophys Acta 1848:1573–1590
Callebaut I, Hoffmann B, Lehn P, Mornon J-P (2017) Molecular modelling and molecular dynamics of CFTR. Cell Mol Life Sci 74:3–22
Farkas B, Tordai H, Padányi R, Tordai A, Gera J, Paragi G, Hegedűs T (2020) Discovering the chloride pathway in the CFTR channel. Cell Mol Life Sci 77:765–778
Mense M, Vergani P, White DM, Altberg G, Nairn AC, Gadsby DC (2006) In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J 25:4728–4739
Li M-S, Demsey AFA, Qi J, Linsdell P (2009) Cysteine-independent inhibition of the CFTR chloride channel by the cysteine-reactive reagent sodium (2-sulphonatoethyl) methanethiosulphonate. Br J Pharmacol 157:1065–1071
El Hiani Y, Linsdell P (2012) Role of the juxtamembrane region of cytoplasmic loop three in gating and conductance of the CFTR chloride channel. Biochemistry 51:3971–3981
El Hiani Y, Negoda A, Linsdell P (2016) Cytoplasmic pathway followed by chloride ions to enter the CFTR channel pore. Cell Mol Life Sci 73:1917–1925
El Hiani Y, Linsdell P (2010) Changes in accessibility of cytoplasmic substances to the pore associated with activation of the cystic fibrosis transmembrane conductance regulator chloride channel. J Biol Chem 285:32126–32140
Wang W, El Hiani Y, Rubaiy HN, Linsdell P (2014) Relative contribution of different transmembrane segments to the CFTR chloride channel pore. Pflügers Arch 466:477–490
Qian F, El Hiani Y, Linsdell P (2011) Functional arrangement of the 12th transmembrane region in the CFTR chloride channel based on functional investigation of a cysteine-less variant. Pflügers Arch 462:559–571
Bai Y, Li M, Hwang T-C (2011) Structural basis for the channel function of a degraded ABC transporter, CFTR (ABCC7). J Gen Physiol 138:495–507
Negoda A, Hogan MS, Cowley EA, Linsdell P (2019) Contribution of the eighth transmembrane segment to the function of the CFTR chloride channel pore. Cell Mol Life Sci 76:2411–2423
Linsdell P (2018) Cystic fibrosis transmembrane conductance regulator (CFTR): Making an ion channel out of an active transporter structure. Channels 12:284–290
Funding
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN/05124–2017).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by PL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Linsdell, P., Irving, C.L., Cowley, E.A. et al. Two positively charged amino acid side-chains in the inner vestibule of the CFTR channel pore play analogous roles in controlling anion binding and anion conductance. Cell. Mol. Life Sci. 78, 5213–5223 (2021). https://doi.org/10.1007/s00018-021-03859-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03859-x