Abstract
The circadian clock exerts an important role in systemic homeostasis as it acts a keeper of time for the organism. The synchrony between the daily challenges imposed by the environment needs to be aligned with biological processes and with the internal circadian clock. In this review, it is provided an in-depth view of the molecular functioning of the circadian molecular clock, how this system is organized, and how central and peripheral clocks communicate with each other. In this sense, we provide an overview of the neuro-hormonal factors controlled by the central clock and how they affect peripheral tissues. We also evaluate signals released by peripheral organs and their effects in the central clock and other brain areas. Additionally, we evaluate a possible communication between peripheral tissues as a novel layer of circadian organization by reviewing recent studies in the literature. In the last section, we analyze how the circadian clock can modulate intracellular and tissue-dependent processes of metabolic organs. Taken altogether, the goal of this review is to provide a systemic and integrative view of the molecular clock function and organization with an emphasis in metabolic tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Function and organization of the circadian timing system
How organisms keep track of time
The rotation of the Earth around its own axis creates a precise 24-h natural light and dark rhythm. This predictability promoted the evolution of internal timekeeping systems that serve to anticipate and adapt to associated changes in environmental demands. Indeed, such internal circadian clock systems can be found from uni to multicellular organisms with an increasing complexity in terms of mechanism and organization [1, 2].
Mammals possess a hierarchical circadian system with a central oscillator, the suprachiasmatic nucleus (SCN), localized in the hypothalamus [3]. The primary role of SCN is to provide subordinate clocks in the brain and peripheral tissues, through several pathways (See "Overview of SCN-driven inputs to peripherel clocks"), with temporal information aligning a multitude of clock-associated biological processes to a single time zone shared by the entire organism in line with the cyclic demands posed by the environment [4,5,6,7,8,9]. Disbalance or disruption of this temporal harmony has been shown to contribute to the development and progression of several diseases [2, 5, 10, 11].
The main synchronizing (or entraining) signal of the circadian clock is light. In the early 2000s, pioneering studies by Ignacio Provencio demonstrated the presence of a novel non-visual photoreceptor in the mammalian retina, melanopsin (OPN4) [12, 13]. OPN4 was shown to participate, together with cone and rod photoreceptors, in the synchronization of the SCN to the environmental light–dark cycle [14, 15]. Two isoforms of OPN4 are expressed in a subset of intrinsically photosensitive retinal ganglion cells (ipRGCs) [12, 16, 17] and are further subdivided into five categories [16,17,18]. OPN4 not only serves as a regulator of circadian rhythms, but it also controls several other light-responsive biological processes such as pupil constriction, melatonin synthesis, glaucoma development, migraine photophobia, and sleep [19]. Upon photon capture by ipRGCs, light information is transformed into electric pulses that travel through the retinohypothalamic tract (RHT), a monosynaptic pathway that innervates the ventromedial (or core) portion of the SCN containing vasoactive intestinal polypeptide (VIP)-expressing neurons. Glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) are released from ipRGC termini in the SCN (Reviewed in [20]). As the core SCN receives direct input from the retina, its photosensitivity leads to a fast increase in transcription of some genes, including the Period (Per1/2) clock genes (Pers), and increased neuronal firing. Single-cell transcriptomics reveal eight cellular subtypes within the SCN, of which five are neurons [21]. There exists intensive communication between the SCN’s core and its dorsoventral (arginine vasopressin-expressing) shell region. The latter has an essential role as the central circadian oscillator, which is beyond the scope of this paper, but the reader is referred to a recent review [22]. Glutamate-induced upregulation of Per gene expression is mainly depended on cAMP-CREB signaling during the night, which results in phase resetting of the molecular circadian clock machinery in the SCN pacemaker [20].
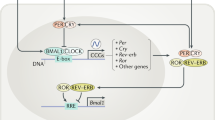
At the cellular level, mammalian circadian clocks are based on interlocked transcriptional-translational feedback loops (TTFLs) comprised of positive and negative arms. In the beginning of the subjective day (circadian dawn), expression of Per (1–3) and Cryptochrome (Cry1/2) genes starts through direct action of the basic helix-loop-helix (bHLH) transcription factor heterodimer, Circadian Locomotor Output Cycles Kaput (CLOCK), or its paralogue Neuronal PAS Domain Protein 2 (NPAS2), and Brain and Muscle ARNT-Like 1 (BMAL1 or ARNTL), acting on E-box cis-regulatory enhancer regions. As gene transcription increases throughout the day, PER/CRY heterodimers accumulate, first in the cytoplasm and then in the nucleus, to represses the activity of CLOCK/BMAL1, leading to a reduction of Per and Cry gene transcription during the subjective night. Critically, PER and CRY protein stability is dependent on post-translational modifications that initiate protein degradation. Casein Kinase 1 δ and ε are responsible for PER protein phosphorylation and degradation while CRY stability is regulated by F-box and Leucine Rich Repeat Protein 3 (FBXL3)-mediated ubiquitination. Once negative-feedback repression is relieved through PER/CRY protein degradation, a new circadian cycle is initiated. In addition to this main TTFL, a secondary loop involves CLOCK/BMAL1 activating the expression of Nuclear Receptor Subfamily 1 group D Member 1 and 2 (Nr1d1/2, also known as Rev-erbα/β) and nuclear receptor Subfamily 1 group F Member 1, 2, and 3 (Nr1f1-3, also known as Rorα-γ). Through competition for binding to orphan receptor response elements (ROREs) in the Bmal1 promoter, REV-ERBs and RORs regulate rhythmic Bmal1 transcription. A third loop consists of BMAL1/CLOCK driving the expression of the PAR-bZip (proline and acidic amino acid-rich basic leucine zipper) transcription factor DBP (Albumin D‑site Binding Protein) that interacts at D-box enhancer regions with the repressor NFIL3 (Nuclear Factor, Interleukin‑3 Regulated, also known as E4BP4), expression of which is driven through the REV-ERB/ROR loop. Such D-box elements are found in the Per promoter regions (Fig. 1).
The molecular mechanism of the circadian clock in mammals. Every 24 h, a complex machinery comprised of genes and proteins undergoes changes in mRNA and protein levels through interlocked transcriptional-translational feedback loops (TTFLs). The reader is referred to the text for a detailed description of this mechanism. Abbreviation of the genes: Per = Period; Cry = Cryptochrome; Clock = Circadian Locomotor Output Cycles Kaput; Bmal1 = Brain and Muscle ARNT-Like 1; CKδ = Casein Kinase 1δ; CK1ε = Casein Kinase 1ε; Nfil3 = Nuclear Factor, Interleukin‑3 Regulated; Rev-erbα/β = Nuclear Receptor Subfamily 1 group D Member 1 and 2; Rorα/β = Nuclear Receptor Subfamily 1 Group F Member 1/2; Dbp = Albumin D‑site Binding Protein
Cyclic chromatin modifications contribute to the stability of the TTFL system and are associated with circadian gene transcription [23]. Through the above-mentioned circadian enhancer motifs hundreds to thousands of tissue-specific clock-controlled genes (CCGs) are regulated in a circadian fashion by the core clock machinery [24]. Collectively, the resulting rhythms of gene/protein abundance allows the organism to keep track of time at the molecular level (reviewed in [9, 22, 25,26,27]). Of note, recent advancements in proteomics suggest that there is only a moderate degree of overlap between rhythmic transcripts and their respective protein products, which argues for a major function of post-transcriptional and translation modifications in circadian timekeeping (reviewed in [28]). As just one example, RNA methylation by N6-adenosine Methyltransferase 70 (METTL3) was shown to affect the period of the molecular clockwork in mice [29].
The molecular clock machinery is not restricted to the SCN. It is, at least in mammals, rather expressed in most—if not all—cells and tissues of the body, thus forming a systemic network of cellular clocks. One may stress that molecular rhythms based on TTFLs are less pronounced or absent in stem cells, but they become detectable as cells differentiate (reviewed in [30]). Several key genes that encode important regulators of biological processes are directly or indirectly affected by the clock gene machinery, a mechanism that ultimately allows biological processes to be within the same time zone [25, 27]. In mice, more than 40% of all protein coding genes display rhythmic transcription in at least one tissue of the body [24].
Organization of the circadian clock network
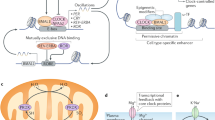
The most accepted system of circadian organization is the “Orchestra Model”—a hierarchical system. In this orchestra, the SCN is considered the maestro, responsible for providing complete information and ensuring coordination among the different band members, i.e., the peripheral clocks. Each musician (tissue/organ) can play its own instrument (regulate biological processes), but the coordination is ensured by the maestro (SCN) [31, 32]. Many researchers argue for a master–slave type of organization between the SCN and peripheral clocks, which is a limited view in face of such complex relationship [33]. Several layers of evidence gathered over the past 20 years have created a consensus in the literature regarding such a hierarchical—top to bottom—control of the SCN over peripheral clocks. Physical disruption of the SCN leads to arrhythmic locomotor activity profiles in rodents kept in constant conditions (DD) or under a light or dark cycle (LD) [3, 34] as well as other important biological functions [35,36,37,38]. However, most of experimental data that led to the creation of this model is based on SCN lesion experiments. Such lesions not only disrupt neuronal connections of SCN but may also affect SCN neighboring connections to other parts of the brain (reviewed in [39]). In addition, evidence arisen from studies in early 2000’s, provided some findings that are difficult to fit into a top to bottom model of organization, such as the fact that timely given food affects peripheral clocks without altering the SCN [40,41,42,43]. Novel experimental tools such as tissue-specific genetic deletion indeed led to changes in the understanding of how the SCN and its surrounding structures regulate circadian rhythms. Mice with Bmal1 deletion in SCN neurons [44, 45] or in the forebrain [46] show rhythmic locomotor activity in LD that is lost in DD [44, 45]. Gene expression data of peripheral clocks of these mice kept in LD also show a rhythmic pattern, while in DD peripheral clock gene expression rhythms are rapidly dampened [44]. Such dampening is paralleled by dampening of corticosterone secretion rhythms [44]. Bioluminescence analyses in tissues from forebrain Bmal1 knockouts (KOs) showed that decreased amplitudes of peripheral clock oscillations upon release into DD was due to impaired cellular rhythmicity and higher phase desynchrony between single cellular clocks. Moreover, time-restricted feeding in forebrain KO mice kept in DD rescues peripheral clock oscillatory responses in the liver, but not in heart, lung, and spleen [46].
The above-described studies demonstrate that in the absence of a functional SCN clock, peripheral clocks still entrain when mice are kept in LD. In zeitgeber free conditions, the SCN plays a vital coordinating role in sustaining internal rhythms. Such data argue for non-SCN regions that receive input from the retina and are able to share such information with other regions in the brain and peripheral organs, thus sustaining internal synchrony (reviewed in [39]). Thus, a new model of organization has been brought forward, the “Federated Model”. It suggests that the SCN is only required to sustain the rhythms of the organism in the absence of zeitgebers or under partially conflicting zeitgeber conditions, which resembles a hierarchical structure. This situation changes when reliable zeitgebers are present, when peripheral organs are able to sustain their rhythms in a SCN-independent fashion, through a tissue-specific combination of zeitgebers, which is expected to allow for more flexible entrainment under complex zeitgeber conditions than a strict hierarchical structure (Fig. 2) (reviewed in [39]).
Current model of circadian network organization in mammals. The suprachiasmatic nucleus (SCN) is regulated by environmental light that is sensed by OPN4 in ganglion cells of the retina. Light information is transformed into electrical stimuli that reach the SCN. Upon SCN synchronization, the organism uses redundant temporal timing cues such as temperature, hormones, and autonomic nervous input to ensure systemic synchronization of biological processes across organs. In addition, behavior can be modulated by a direct effect of environmental light, the SCN, or by food availability which, in turn, can directly affect the molecular clocks of peripheral organs
Chronodisruptive environment and its impact on the clock
Our current society’s living conditions are strikingly different from the early days of Homo sapiens [47]. With the implementation of widespread illumination in the late 1870’s, the presence of light during the evening became a reality. One may not overcast the advancement that lighting made for mankind, but it should be stressed that its putative deleterious effects may have been overlooked in the past decades. In this new environment in which light is strongly present, an important consequence takes place: reduction of the regulatory role of the light/dark cycle as a circadian zeitgeber. In this sense, social cues have increased their effect on human rhythms and some authors consider that we are currently living in a constant social jetlag paradigm (reviewed in [11]).
Advancements made in the food industry during the last decade have led to a state of 24-h food abundance. Moreover, modern food items are often loaded with fat and sugar. As an additional confounder, our current lifestyle is often associated with a lack of exercise as well as working in shifts [2, 11]. Therefore, the conditions in which our biological clock evolved are vastly different from the ones we face now. The biological clock is programmed to anticipate physiological events based on predictable environmental factors that include mainly light, temperature, food, and activity. However, our current society’s lifestyle poses as a challenge to this system since we have a combination of factors that negatively affect our clock. Overall, the presence of light at night, continuous availability of high-fat and -sugar food, which can be associated with a sedentary lifestyle, and the presence of night shift works, result in a condition of chronodisruption of which we currently do not fully comprehend the consequences to human’s health.
The last decade witnessed a worldwide movement to replace traditional lamps for LEDs due to their high energy efficiency [48]. However, such LEDs display a sharp blue emitting peak that is able to influence our circadian system via OPN4-detection in the eye [49] and may promote eye-related pathologies [50]. However, there are a scarce number of studies with appropriate control to evaluate the effects of LED vs. traditional lamps, which is acknowledged by the European Scientific Committee on Health, Environmental and Emerging Risks (SCHEER). However, SCHEER also acknowledges the effects of LEDs on the circadian system via melatonin suppression in controlled studies. Due to insufficient data in humans and appropriated controls in the studies, SCHEER reached to the conclusion of no evidence of direct adverse health effects from LED emission devices [51]. Nevertheless, SCHEER also recognizes that LED technology is still growing and a closer attention to its long-term effects is required [51].
The best example of chronodisruption in humans are shift workers. An increasing number of epidemiological studies show an association of shift work with the development of several diseases (reviewed in [52, 53]). Remarkably, very recently the International Agency for Research on Cancer (IARC) kept the same recommendation made in 2007 that shift work in humans is “probably carcinogenic to humans”, evidence grade 2A, i.e., with sufficient data in experimental models but limited in humans, mainly due to a higher variability between the studies [54, 55]. Moreover, studies that combine satellite images with epidemiological data suggest a correlation between light at night (LAN) exposure with increased incidences of breast cancer—but not with colorectal, larynx, liver, and lung cancers [56]—as well as overweight and obesity [57]. Nevertheless, conclusive data about LAN effects are still pending. Despite the lack of hard evidence for the deleterious effect of shift work and LAN in humans, it is clear that the conditions of our society are completely different from the ones in which biological clocks evolved [1, 47].
Based on such knowledge, it is tempting to link the increasing incidence of several diseases such as metabolic, cardiovascular and neurological disorders, and cancer in humans to a possible disruption of the circadian clock (reviewed in [1, 2, 10, 47, 58, 59]). Ultimately, the consequences of chronodisruption to human health are still being comprehended—it is an ongoing field of investigation. However, the more we understand the long-term consequences, the better policy makers can act, which could potentially prevent a substantial loss of life and provide a better life quality for large parts of the population.
Communication between central and peripheral clocks: a one-way street?
Overview of SCN-driven inputs to peripheral clocks
The pathways used by the SCN to relay temporal information to the organism comprise of complex and partly redundant signals which can be divided into four categories: sympathetic and parasympathetic nervous stimuli, hormonal outputs, feeding-fasting rhythms, and circadian oscillations of core temperature rhythms (Fig. 2). SCN efferent projections have been shown to terminate in several brain regions such as sub-paraventricular zone, the preoptic area, the nucleus of the stria terminalis, the lateral septum, the dorsomedial hypothalamus, the arcuate nucleus (ARC), and the paraventricular nucleus (PVN reviewed in [6, 8, 31]). In addition, the SCN receives input from hypothalamic and extra-hypothalamic regions, which also are important for regulating SCN physiology [6, 60].
The first pathway is autonomic output from the SCN that leads to rhythmic clock gene expression [61]. However, autonomic denervation is not required for rhythmic gene expression in the liver [62], thus arguing for additional routes of synchronization. Interestingly, SCN-driven sympathetic outputs have been shown to decline with aging [63].
A second pathway is comprised of hormonal outputs mainly comprised of the pituitary–adrenocortical axis (HPA), specifically via glucocorticoids. Corticoliberin (CRH) secretion by the PVN exerts a control over the rhythmic release of adrenocorticotropic hormone (ACTH) in the pituitary. Through downstream signaling by the pituitary, glucocorticoids are rhythmically released by the adrenal cortex peaking at the beginning of the active phase [35, 64, 65]. Glucocorticoid action takes place through mineral (MR) and glucocorticoid receptors (GR), whose expression is widely found in peripheral tissues [66]. It is worth mentioning that the promoter region of the Per genes contains glucocorticoid response elements (GREs) whose activation leads to gene transcription and provides a molecular basis for glucocorticoid action in the circadian machinery [67]. Another classical synchronizing hormone is melatonin, which (known as the hormone of darkness) is secreted exclusively during the night in both nocturnal and diurnal species. Upon light exposure, the SCN sends inhibitory information to the PVN region, leading to suppression of melatonin synthesis. In the dark, a glutamatergic activation of the PVN leads to norepinephrine release in the pineal gland and subsequent melatonin synthesis (Reviewed in [68]). Melatonin conveys temporal information to peripheral organs through its interaction with its G-protein coupled receptors (MTNR1A/B or MT1/2) found in peripheral organs (reviewed in [68, 69]). Melatonin suppression by light at night (LAN) as result of modern lifestyles [1] is associated with several diseases, probably through its disruptive effect on the circadian clock network [70, 71].
A third pathway involves feeding-related activities. Temporal food restriction is an important zeitgeber for metabolic tissues, being able to synchronize local clocks while exerting few effects on the SCN [41, 42, 72]. Of note, though, combining timed feeding with hypocaloric diet regimes can affect SCN activity and SCN-driven outputs such as locomotor activity, melatonin synthesis [73, 74]. In rodents, very restrictive feeding regimes also led to alterations in SCN clock gene expression, though SCN outputs were not evaluated in these studies [75, 76].
The last pathway used by the SCN is internal temperature rhythms. Thermoregulatory coordination processes are centralized in the hypothalamic preoptic area in which the median preoptic nucleus is a key player [77, 78]. Core body temperature increases and decreases before the onset of the active and resting phases, respectively. Such anticipatory regulation is controlled by the SCN [79]. Along the day, core temperature is known to be rhythmic oscillating from 36.5 to 38.5 °C with a mean of 37 °C in humans and rodents [80,81,82]. Mounting evidence from in vitro studies has demonstrated that tissues and cells of peripheral clocks are able to respond to cold or warm temperature when presented as a short pulse or cycles. Thus, diurnal variations in body temperature are assumed to play an important role in peripheral clock synchronization [83,84,85,86,87]. The receptors responsible for detecting thermal energy in peripheral tissues are still elusive. However, the large family of transient receptor potential (TRP) channels [88,89,90] and, more recently opsins, whose thermo-detecting capabilities have been demonstrated in mammals, are interesting candidates that may detect temperature fluctuations to reset molecular clocks [83, 91, 92].
Overview of peripheral inputs affecting the SCN
The SCN is considered largely resistant to potential synchronizing factors other than light. For instance, glucocorticoids display no or only indirect effects on the SCN due to an absence of GR receptors in this area [93, 94]. The same holds true for temperature changes since the SCN is able to compensate these effects [86, 95]. The insensitivity of the SCN to synchronizing factors is important for its role as a central oscillator, though this does not mean that it cannot integrate feedback factors to adapt systems rhythms.
One of such factors, leptin, the satiety hormone, displays peak blood levels at night in nocturnal rodents, which are controlled by SCN-driven sympathetic innervation of adipose tissue [96]. In vivo leptin injections at the beginning and the end of the diurnal activity phase do not affect locomotor activity of mice kept in either LD or DD conditions. However, leptin augments the phase-shifting effects of light pulses during subjective night specifically in females [97]. Vice versa, in leptin deficient ob/ob mice, leptin administration normalizes photic responses at the behavioral and molecular level [98]. Another important metabolic hormone, ghrelin that is synthetized by the oxyntic gland cells of the stomach participates in the anticipation of feeding time by activating its receptors in the ARC [99, 100]. In addition, ghrelin positive neurons in the hypothalamus receive direct input from the SCN [101]. In vivo experiments reveal that ghrelin, when given to animals kept under an ad-libitum diet, does not affect locomotor activity. However, upon food deprivation ghrelin analogs phase advance locomotor activity in mice in DD [102]. Of note, the SCN is resistant against insulin-induced clock resetting [103].
Hypocaloric restricted feeding regimes display a strong effect on the SCN [73, 74]. In addition, long-term high-fat diet (HFD) conditions influence the SCN of male mice [104, 105], but not of females [106, 107]. One week-long HFD does not affect SCN rhythms but disrupts liver clock regulation [108]. Remarkably, the starvation signal, fibroblast growth factor 21 (FGF21), a liver-born molecule, leads to decreased systemic insulin levels and locomotor activity, and increased corticosterone, which represents a classical starvation response. Such responses were dependent on the presence of β-klotho in the SCN and in the dorsal vagal complex of the hindbrain [109]. Moreover, β-hydroxybutyrate (β-OHB), a liver-borne molecule, has been implicated as a player in food-anticipatory activity (FAA) response via a putative interaction with brain regions, which are still elusive [110].
Melatonin, whose receptors are present in the SCN, has important effects on neuronal firing and clock gene expression in the central pacemaker [111,112,113]. Additionally, increased blood pressure was shown to affect neuronal activity in the nucleus tractus solitarius (NTS), but also in the SCN. Interestingly, the SCN receives direct projections from the NTS and plays an important integrative and regulatory role in blood pressure circadian control [114]. Physical exercise does not affect the SCN, but it resets many peripheral clocks [115]. More recently, the presence of a non-metastatic melanoma tumor in mice was shown to reduce Bmal1 and increase cFos gene expression in the SCN compared to control animals, suggesting that molecules released from the tumor may affect the SCN as well peripheral tissue clocks [116].
Relative autonomy of peripheral tissue clocks
Global knockout mice have allowed a substantial increase in our knowledge regarding gene function. However, with regard to the circadian clock network, this strategy is limited since gene deletion is global and do not allow dissecting the effects of specific tissue clocks on physiological functions. Such limitation was circumvented by tissue-specific gene knockout strategies such as the Cre/loxP system, which can also be conditional, i.e., gene deletion may be induced at a specific timepoint of development (Reviewed in [117]). Regarding the molecular clock, only the Bmal1 deletion results in loss of rhythms as other clock genes are functionally compensated by their paralogs which makes dual knockouts necessary, which is the case for Per and Cry genes (reviewed by [118]). So far, an increasing number of studies using global and tissue-specific knockouts has allowed our current understanding regarding the functional organization of the clock network in both physiological and pathological scenarios.
Early organ transplantation experiments between clock gene mutant and wild-type animals have been key to understand circadian organization at the network level [3, 65]. More recent advancements in molecular biology allowed studying the molecular clock in a single organ while the others remain without a functional clock machinery. In these landmark studies, using a conditional gene trap strategy, in mice lacking Bmal1 in all tissues, Bmal1 expression and, thus, clock function is restored upon Cre expression in either liver or the epidermis of the skin [119, 120]. In the liver clock rescue mice, glycogen synthesis and nicotinamide adenine dinucleotide (NAD +) salvage production are rhythmic, but only when mice are kept in LD. Thus, the liver clock is able to sustain its autonomy despite the absence of functional clocks in the rest of the organism—including the SCN. Rhythmic hepatic lipid and xenobiotic metabolites are not restored, though. In line with this, compared to wild type mice, reconstitution of the molecular clock of the liver rescues rhythmicity in approximate 10% of gene transcripts and 20% of metabolites. These data show that most rhythmic processes of the liver are not autonomous and dependent on systemic time cues that only exist in an intact circadian organism. Along this line, liver clock restoration does not revert the reduced life span of global Bmal1 KO mice [119]. In an accompanying study, clock gene restoration of the epidermis yielded similar findings to the ones described above. Upon epidermal clock rescue, approximately 15% of transcriptional rhythmicity is restored. Again, all rhythms are completely lost in DD [120]. Overall, local tissue clocks in the epidermis controls basic tissue functioning, i.e., epidermal turnover. Epidermal clock restoration does not rescue the reduced life span of Bmal1 KO mice, but it inhibits premature skin aging [120].
This new strategy has opened a new investigative field, which will allow the precise contribution of each organ system to the overall circadian organization. Importantly, both studies do not establish whether the arrhythmicity found in the clock rescued liver or skin in DD is due to complete loss of rhythms at single cell levels or due to a loss of coupling (i.e., circadian communication between cells), which ultimately leads to an apparent loss of rhythms. At the same time, it emphasizes the key role of light in clock resetting—even at the peripheral tissue level. One may argue that mouse skin cells, which are known to be photosensitive [121,122,123,124,125] could be responsible for synchronizing the local clock in vivo. Indeed, such concept has been proven as neuropsin (OPN5) participates in light-induced clock gene synchronization of the skin [124]. This does obviously not apply for liver, though. Studies from our lab had previously shown that light synchronizes peripheral clocks in the absence of a functional SCN, thus suggesting that other brain regions that receive light input via retina may ultimately sustain circadian rhythms of the organism [44, 45]. In summary, these findings argue for a less SCN-centric organizational view of the circadian clock system, which agrees with the federated model of organization [39].
Very recent experiments provide evidence that liver or fibroblasts from Bmal1 KO mice, when cultivated ex vivo and synchronized with dexamethasone, indeed display robust rhythms in gene and protein expression for days. The authors also ruled out a putative compensatory function of Bmal2 (Arntl2). Interestingly, Bmal1 KO cells displayed almost no overlapping rhythmic transcripts and proteins compared to wild-type cells. Indeed, in the absence of Bmal1 a new set of transcription feedback loop is recruited controlling novel and non-canonical molecular rhythms [126].
Taken altogether, systemic cues are important to drive circadian rhythmicity of liver, skin, and likely all other organs. However, local clocks can sustain rhythmicity of some biological processes even in the absence of a functional circadian network. It will be of great importance to identify the mediators of circadian network timekeeping as they make great targets for chronotherapies. In addition, a novel non-canonical clock was reported in the absence of Bmal1. Such processes may be of importance in diseases in which circadian disruption alleviates physiological control by the clock machinery.
Putative mediators of inter-tissue communication
As described previously, we have uncovered how SCN signals to periphery as well as how periphery may affect SCN functioning. In this section, our aim is to provide an overview of putative signals released by peripheral organs and how they may affect clock gene functioning of peripheral tissues.
Based on a decentralized circadian organizational view, one may expect the presence of redundant signals that arise from: (1) the SCN to periphery; (2) periphery to SCN, (3) and between peripheral organs. Collectively, these pathways sustain the circadian network organization (Fig. 3). Twenty-four hours long metabolomic data of SCN, medial prefrontal cortex, liver, muscle, sperm, WAT, BAT, and serum demonstrated an extensive communication network between organs to sustain coherence of the temporal metabolic pathways. Remarkably, such coordination and inter-tissue metabolite correlation and coordination is lost and rewired by high-fat diet. Interestingly, while some tissues show partial gain or loss of metabolites rhythms, the serum metabolites are the most affected, i.e., being the most affected (loss of correlation) under HFD [127].
Bidirectional communication between central and peripheral tissue clocks. Temporal cues controlled by the central pacemaker (SCN) and other CNS clocks such as glucocorticoid and melatonin secretion, autonomic inputs, and body temperature are known to affect the molecular clocks in peripheral organs. At the same time, peripheral clocks, through molecules like leptin, ghrelin, FGF21, and adiponectin can feed back on the SCN and other brain region clocks. The overall result of this entangled communication network is an integrated rhythmic control of behavior and metabolic outputs. In this figure, it is didactically represented the phase (day or night) in which each factor has its highest value in humans
The identification of putative players responsible for regulating the molecular clock of peripheral organs is still largely elusive. Indeed, the role of several gastrointestinal peptides such as cholecystokinin (CCK), gastrin, ghrelin, glucose-dependent insulinotropic polypeptide (GIP), motilin, neurotensin, neuropeptide Y family, secretin, and VIP in regulating circadian rhythms have been suggested but experimental evidence is still lacking (reviewed in [128]). On the other hand, oxyntomodulin (OXM), a glucagon-like hormone, administration to liver explants was shown to affect the molecular clock and carbohydrate-related CCGs as well as when given in vivo. Interestingly, blockade of OXM suppresses food-induced molecular clock alterations in the liver—but not in the SCN—thus placing OXM as a direct link between food-related effects in hepatic circadian clock [129]. Ghrelin administration to steatotic liver was shown to restore circadian rhythmicity in in vitro and in vivo in an mTOR-dependent fashion [130]; however, the role of ghrelin in a physiological setting is still unknown.
Insulin and glucose are classical players in modulating the molecular clock of metabolic and non-metabolic organs. For instance, insulin and insulin-like growth factor-1 (IGF-1) affect both the phase and amplitude of clock genes in in vitro, ex vivo, and in vivo through increased PER transcripts of liver and kidney. Such response was not dependent on the classical clock genes and the SCN was insensitive to the insulin-dependent effects [103]. It has been suggested that insulin is an important player in food-induced clock gene alteration in the liver as streptozotocin-treated animals, i.e., without insulin production, are not affected by food regimes while exogenous insulin rescued the effects of food in the hepatic clock [103].
Indeed, insulin has been shown to signal through phosphatidylinositol 3-kinase (PI3K) and Forkhead box class O3 (FOXO3) signaling to sustain liver rhythms in a Clock-dependent fashion [131]. Insulin has also been shown to phosphorylate BMAL1 in a protein kinase B (AKT)-dependent process, which results in BMAL1 dissociation from the DNA, and consequently, a reduction in its transcriptional activity [132]. Moreover, cAMP Responsive Element Binding Protein 1 (CREB)/ CREB regulated transcription coactivator 2 (CRTC2) role has been suggested in liver clock synchronization during fast and feeding. During fast, glucagon activates CREB/CRTC2 that ultimately induces Bmal1 transcription while insulin, on the other hand, suppresses Bmal1 expression by inhibiting CREB/CRTC2 activity [133]. Interestingly, CRTC2 has been shown to regulate hepatic gluconeogenesis in a cooperative fashion with glucocorticoid hormones and its receptor and glucagon via CREB-dependent pathway [134]. Whether CRTC2-dependent interaction with glucocorticoid, whose role as a synchronizer of the hepatic clock is established [135], is still elusive.
Importantly, mTOR signaling has been suggested as a vital pathway through which peripheral clocks use to adjust their local circadian rhythms. In both central and peripheral clocks, mTOR activation results in shorter oscillatory profile, i.e., it speeds up the clock while mTOR inhibition results in the opposite effect [136]. Therefore, mTOR pathway seems to be a broad signal hub for adjusting the molecular clocks; however, the identity of players that modulate this pathway is largely unknown. Nevertheless, such widespread pathway may play useful in restoring circadian functioning in pathological conditions.
Taken altogether, the identification of peripheral clock regulators that are not directly driven by the SCN, but rather by peripheral clock themselves is ongoing field of investigation, and there are many gaps of knowledge. Indeed, one may suggest a complex and redundant communication network through which peripheral clocks communicate with each as elegantly shown by Dyar and colleagues (2018) [127]. Comprehending the role of each hormone, peptide, and/or metabolite will prove an exhausting task; however, it must be emphasized that the discovery of such players and pathways would be crucial for the development of novel pharmacological targets for metabolic diseases.
Clock-metabolism crosstalk
Cellular processes
The importance of the biological clock as a temporal regulator and coordinator of complex biological processes of tissues and systems can be appreciated when key biological processes of a particular organ are comprehended. Extensively revising each biological process is beyond the scope of this review. However, in the next lines, we will highlight how the molecular clock is of importance to ensure metabolic homeostasis by controlling some important intracellular processes as well as biological processes of metabolic organs.
One of the most important clock-regulated processes is the information flow from DNA to proteins. In recent years, a close association between clock proteins and histone modifications has been unraveled contributing to the regulation of circadian rhythms at the molecular level. Specific cyclic chromatin transitions happen along 24 h and are associated with changes in transcriptional activity [23, 137,138,139]. Among different types of histone modification, acetylation is the most studied with regard to circadian rhythms [23].
As for the mRNA level, several lines of evidence show that most transcripts (nascent and processed RNAs) are not transcribed in a circadian fashion, thus ruling out a concept that most gene rhythms would be driven by E-box mediated transcription. Indeed, abundance of only approximate 25% of cycling mRNA transcripts are controlled by de-novo transcription, thus suggesting that post-transcriptional regulatory mechanisms are critical to generate expression rhythms for the remaining 75% of mRNAs [138, 140]. On the other hand, recent mathematical modeling has suggested that a lesser faction (~ 35%) of rhythmic mRNAs are regulated at the post-transcriptional level [130]. mRNA processing such as capping, splicing, polyadenylation, and cytoplasm exportation have been shown to be clock regulated, thus contributing to overall circadian organization (reviewed in [141]). Another important regulatory step is miRNA-mediated mRNA degradation. In this sense, a liver-specific Dicer knockout demonstrated that in the absence of most miRNAs, the hepatic clock machinery is still functional and shows modest alterations in gene expression in terms of phase and period. Such findings demonstrate that miRNAs may not be major driving forces of mRNA rhythms, but rather miRNAs are a fine-tuning tools of rhythmic transcriptomes [142], which is further supported by other experimental studies [143, 144].
Many studies have highlighted an important lack of concordance between rhythmic mRNA and protein levels, thus suggesting that many rhythmic proteins do not undergo an oscillation at the transcript level [130, 145, 146]. This may be explained by oscillatory rhythms in ribosome biogenesis and translation initiation complex formation [147,148,149] which may overcome the lack of oscillation at the mRNA level. Additionally, recent studies have revealed further post-translational modifications and their regulatory role in circadian timekeeping. Phosphoproteome analyses from liver revealed that 25% of phosphorylation sites and 40% of phosphoproteins are regulated in a clock-dependent manner [145]. In the murine hippocampus, approximately 2% of proteins and 5% of phosphorylation sites exhibit a circadian profile; however, in this study, no clock proteins were detected [150]. In mouse liver, 8% of proteins are rhythmic and are associated with immune responses, cell cycle regulation, lipid and glucose metabolism. Interestingly, almost 3,500 ubiquitination sites were found on 1144 proteins, which were enriched during daytime compared to night [151]. For a detailed view of post-translational processes involved circadian clock function, the reader is referred to a recent review [152].
Taken altogether, recent evidence highlights the various layers of circadian control, starting from DNA accessibility to transcription factors, a histone dependent process, which is followed by RNA transcription. Upon mRNA synthesis, several regulatory (post-transcriptional) steps need to be met, and most of them are subject to circadian control. Finally, information is translated from mRNA to protein, which also undergo several post-translational modifications that directly affects biological function. The increasing view that de-novo transcription accounts for just a fraction of rhythmic gene expression, a long-standing concept in the field, associated with the lack of overlap between rhythmic genes and proteins, place post-transcriptional and translational modifications as important regulatory steps of the circadian clock (Fig. 4), whose role and functions are still being understood.
The classic cellular information flow from DNA to RNA and to protein is subject to circadian control at all levels. This rhythmic biological information flow contributes to the circadian control of cellular physiology and downstream biological processes. Among these, we highlight DNA repair, oxidative stress, and cell cycle regulation
The cell cycle consists of three main processes that include a DNA replication step (S phase) that is followed by a stage of mitosis (M Phase), and in between growth stages (G1 and G2) in which cells prepare themselves for the next division. In G1, cells prepare for DNA replication by increasing protein synthesis and cell growth. In G2, growth-related processes also take place in addition to the DNA damage check point, a fundamental step to avoid wrong, potentially deleterious, information to be passed on to daughter cells. Sequentially speaking, dividing (non-quiescent) cells start increasing their size (G1), which is followed by DNA replication (S Phase) and another growth phase (G2) that is followed by mitosis (M Phase). Quiescent cells (G0) may enter the cell cycle depending on a series of environmental factors. Senescent cells, on the other hand, are terminally differentiated and cannot re-enter the cell cycle. The progression through each stage is strongly controlled and dependent on a series of activations of cyclin-dependent kinases (CDKs) that form specific complexes with cyclins (CCNs) for each cell cycle transition [153,154,155]. Elegant in vitro studies have demonstrated that in free-running conditions there is a robust coupling between cell cycle and circadian clock. Interestingly, in active dividing cells a shortening of clock period happens when compared to non-dividing cells, thus suggesting an influence of the cell cycle on the molecular clock [156, 157]. On the other hand, in synchronized cells, two major events take place: a fraction of cells remains phase locked, i.e., one cell cycle per one clock cycle while another sub population displays three cell cycles for each two clock cycles [157]. Taken together, these studies suggest that in absence of synchronizers (free-running conditions), the influence of the cell cycle on the molecular clock is dominant. On the other hand, in synchronized conditions the circadian clock displays a dominant role over the cell cycle. Some researchers have suggested that the molecular clock is able to “gate”, i.e., allow cell cycle progression [158], a concepted that has been challenged [156, 157, 159]. The molecular clock exerts its effects on the cell cycle either by controlling transcription of key regulatory genes or by protein–protein interaction (reviewed in [160]). In this line, it is not surprising to realize that disruption of the circadian clock is in fact correlated to a loss of cell cycle progression control, often seen in cancer [161] [162,163,164].
In an elegant and complex process, DNA repair can revert DNA damage caused by a myriad of genotoxic agents. There are several types of DNA repair in mammals such as direct repair (by alkyl transferases), base excision repair (BER, by glycosylases and AP endonucleases), double-strand break/crosslink repair, and nucleotide excision repair (NER) [164]. Available evidence suggests that NER is strongly controlled by the clock. NER-dependent DNA repair is carried out by six factors: replication protein A (RPA), Xeroderma Pigmentosum Complementation Group A, (XPA), Xeroderma Pigmentosum Complementation Group C (XPC) complexed with RAD23 Homolog B (HR23), Transcription factor II Human (TFIIH), Xeroderma Pigmentosum Complementation Group G (XPG), and Xeroderma Pigmentosum Complementation Group F (XPF). For a detailed view of this process the reader is referred to in-depth reviews [164,165,166]. In pioneering studies led by Aziz Sancar’s group, NER mediated activity was shown to be rhythmic in brain [167] and liver, but not in testis [168] and skin [169]. In these tissues, XPA was the only NER-related protein to show an oscillatory profile, in antiphase to CRY1 [167,168,169]. In the absence of CRY1/2, such rhythmic profile of XPA was abolished in liver and skin [168, 169]. In terms of functional activity, excision repair of cisplatin adducts in the liver and UVB-induced DNA damage are highest in the afternoon (4–5 pm) and lowest in the morning (4–5 am), thus highlining important findings for chronomodulated therapies [168, 169]. Moreover, UVB-induced erythema is maximal in the morning vs. exposure in the afternoon in mice, which is associated with reduced DNA repair in the early morning compared to the afternoon [170]. Indeed, such concept has been translated recently to humans as UVB-induced erythema was shown to be milder in the morning compared to evening exposures [171]. Some studies have suggested that BER-dependent activity may also be influenced by the molecular clock. BER-mediated DNA repair activity has also been suggested to display a rhythmic profile in humans as the expression of 8-oxoguanine DNA glycosylase (OGG1) is rhythmic. Oxidative DNA repair in lymphocytes is higher in the afternoon compared to the morning. In vitro experiments show that Bmal1 knockdown leads to loss of rhythmic expression of OGG1 [172]. In addition, in hepatocytes, BER activity, via N-methylpurine DNA glycosylase (MPG) protein expression, is rhythmic and depends on CLOCK [173].
Controlled generation of reactive oxygen and nitrogen species (ROS/RNS) in a cell is an important step for cellular signaling processes since these molecules may act as secondary messengers. Disruption of this rigid control may result in increased ROS levels, which may give rise to oxidative stress, damage macromolecules, and ultimately promote apoptosis or necrosis (reviewed in [174]). Cellular ROS is predominantly generated as superoxides because of mitochondrial oxidative phosphorylation. Detoxification of ROS takes places via a complex set of enzymes such as superoxide dismutases (SODs), catalase, glutathione peroxidase (GPx), and free radical acceptor molecules like peroxiredoxins (PRDXs), glutathione (GSH), or thioredoxin (TRX) (reviewed in [174, 175]). Without a proper control of ROS/RNS generation and detoxification, the consequences for cellular fate can be disastrous. Indeed, chronic oxidative stress has been implicated in the development of several diseases such cardiovascular, endocrine, aging, neurodegenerative, cancer, and many others [174, 176, 177]. Since ROS is linked to cellular metabolic state, which itself is known to undergo an oscillatory rhythm, the generation of ROS and the expression of detoxification enzymes have been shown to oscillate along the day in mammals [178,179,180,181,182,183]. Indeed, the presence of E-box elements in promoter regions of several detoxification enzymes has been shown in humans and mice, thus suggesting a possible route of ROS control via transcriptional activity by the molecular clock [184]. Interestingly, circadian control of oxidative defenses was shown to take place in absence of transcriptional activity in red blood cells [185]. For a more in-depth information, the reader is refereed to excellent reviews on this topic [186, 187].
Metabolic tissue circadian functions
In the last section, we will provide an overview of clock regulated biological processes in metabolic organs (Fig. 5) and some recent advancements made in the field.
White adipose tissue (WAT) is the most abundant type of adipose tissue in mammals and has traditionally been associated with its energy storage capacity in form of triacylglycerol (TAGs). In this process, TAGs are released from WAT via lipolysis to generate free fatty acids (FFAs) and glycerol, which are taken up by other tissues to generate energy [188]. The WAT molecular clock has been shown to control the above-mentioned process of lipolysis [189, 190]. In addition to its classical energy storage capacity, it has become increasingly recognized that WAT is an active endocrine organ able to synthesize several biologically active molecules, which may regulate homeostasis [191]. Several studies have demonstrated rhythmic expression of clock genes in WAT of different origins in mice [189, 192,193,194] and humans [195,196,197]. Moreover, microarray and transcriptomic analyzes have demonstrated that hundreds of genes show an oscillatory profile in mice [24, 193, 198] and humans [196, 199, 200], many of which are involved in key physiological processes such as lipolysis, adipogenesis, and energy conversion. The role of the molecular clock in regulating WAT physiology can be clearly seen in clock gene knockout mice. Global deletion of Bmal1 or Rev-Erbα inhibits [201, 202] while Per2 or RORα/γ knockouts favor adipogenesis [193, 203]. Along this line, peroxisome proliferator-activated receptor gamma (PPARγ), a classical clock-controlled factor, plays a pivotal role in adipocyte differentiation [204, 205]. Interestingly, rhythmic expression of core clock genes and Pparγ is severely dampened in experimental models of HFD [105]. However, female mice are protected from the deleterious effects of HFD [106, 107] which seems to be associated with smaller effects on the molecular clock machinery [206]. In the clinical setting, the therapeutic value of PPAR agonists is clear as fibrates (targeting PPARα) are known to reduce plasma lipids while thiazolidinediones (PPARγ) improve insulin sensitization in diabetic patients [207]. Moreover, clinical investigation of dual or pan-PPAR activators for treating metabolic-related disorders are currently being evaluated [207]. Global clock gene knockout is often associated with altered WAT functioning and body weight, which is gene-dependent (reviewed in [208]). As data from global knockout mice suffer from several limitations, the physiological role of each clock gene in a particular organ might be over- or underestimated. In recent years, adipocyte-targeted clock gene deletion has rendered interesting results. Adipocyte protein 2 (aP2) gene-Cre driven Bmal1 removal leads to increased food consumption and body weight—regardless of diet type. In addition, mice with targeted Bmal1 deletion in WAT show altered polyunsaturated fatty acid serum levels, which affects central mechanisms of appetite regulation in the hypothalamus. Indeed, non-obese patients display rhythmic serum levels of leptin and adiponectin [209], which are dampened in obese subjects [210]. A specific WAT Bmal1 deletion under adiponectin (Adipoq) Cre control has a mild phenotype and regular body weight on standard diet, but displays a higher probability to become obese under HFD [190]. WAT of mice with targeted SCN clock gene disruption show arrhythmic expression of genes related to lipid and carbohydrate metabolism. However, in this model genes involved in immune response gain rhythmicity in the absence of an SCN clock [198]. In humans, single nucleotide clock gene polymorphisms (SNPs) have been associated with development of metabolic disorders (reviewed in [211]). Moreover, loss of clock gene rhythms in type-2 diabetic patients has been shown when compared to healthy individuals. Interestingly, in diabetic patients, loss of rhythms in lipolysis was also found [200].
Brown adipose tissue (BAT) is a specialized thermogenic organ that can convert chemical energy into heat and recently. BAT has recently moved into the focus of translational research due to its putative role in fighting obesity and diabetes [212]. BAT thermogenic activity is triggered by cold stimuli in an adrenergic-dependent pathway, which results in lipolysis. FFAs are transported into mitochondria in which they undergo β-oxidation. In BAT, this process does not lead to energy generation, but rather results in non-shivering heat generation. This is possible due to uncoupling protein 1 (UCP-1), which creates proton leaks in the respiratory chain, thus generating heat instead of energy [213]. Interestingly, circadian transcriptome analyzes have revealed that approximately 10% of protein-coding genes [24] as well as approximately 40% of nuclear receptors in BAT oscillate within a day [214]. Glucose levels, FFAs uptake, and clearance of serum lipids in BAT is rhythmic [215, 216]. Interestingly, mice exposed to a cold stimulus at 5 a.m. respond better compared to controls exposed at 5 p.m. due to reduced Rev-erbα expression in the morning. Remarkably, loss of Rev-erbα improves cold tolerance. Rev-erbα knockout mice show increased UCP-1 levels and loss of rhythmic body temperature and BAT activity [217]. In Rorα knockout animals increased UCP-1 levels and browning of WAT is found in addition to a higher ex vivo respiration in both BAT and WAT compared to control [218]. On the other hand, Per2 knockout mice are cold-sensitive due to impaired heat shock factor 1 (HSF1) signaling [219] while Bmal1 knockout mice show no difference in cold-stimulation responses compare to controls. An interesting study has demonstrated that increased light exposure (16 h per day) vs. standard light regimes (12 h light per day) leads to increased adiposity with no effects on food intake or locomotor activity. Such response is associated with decreased sympathetic input to BAT and reduced FFA uptake [220]. It is still elusive which players that regulate the proper functioning of the circadian clock machinery in BAT. For instance, TRP channels, in particularly TRPV1 and TRPM8, have been shown to affect the molecular clock since in Trpv1 and Trpm8 knockout mice, oscillatory profiles of clock genes are lost or disrupted [89, 90]. Moreover, β-adrenergic receptors are necessary for basal and cold-induced thermogenic responses, but are dispensable for molecular clock function [221]
The endocrine pancreas is a complex organ comprised of different cell types that regulate glucose homeostasis in a daily basis. There are two cell types of major importance in this process: insulin-secreting β-cells and the glucagon-secreting α-cells. It is widely established that insulin and glucagon levels are circadian independent of feeding regimes [222, 223]. Initial studies suggested an important role of the SCN in regulating daily rhythms of insulin and glucagon. However, such experiments were based on surgical SCN lesions, whose limitations are highlighted in "Organization of the circadian clock network". Several studies have demonstrated a functional circadian clock in pancreas responsible for controlling insulin secretion in a daily fashion in different mammalian models [224,225,226] including humans [227]. Amongst the clock genes, an interesting role has been suggested for Dbp and Thyrotroph Embryonic Factor (Tef) in pancreatic clocks [228, 229]. Omics approaches have shown that approximately 30% of the ß-cell transcriptome show a circadian oscillation and were involved in insulin-related vesicle trafficking and membrane fusion processes. Remarkably, transcriptomic data of ß-cell-specific Bmal1 knockout mice show rhythm blunting for genes involved in exocytosis [230]. In vivo and in vitro transcriptomic analyzes of α- and β-cells reveal distinguished gene programs throughout the day in each cell type, which are believed to contribute to the temporal orchestration of insulin and glucagon release [231]. Neonatal pancreatic clocks are not fully functional at early stages and miRNAs were suggested to participate as regulators of emerging circadian clock functioning in the post-neonatal phase [232]. Global Clock deletion results in increased glucose levels and impaired glucose tolerance. Such effects are absent in young (3 months old) mice, but become evident in older (8 months old) animals [224]. Ex vivo stimulation with glucose of isolated pancreatic islets shows a marked reduction in insulin secretion in both young and old Clock knockout mice [224]. The global absence of Bmal1 also yields impaired glucose-induced insulin responses [224]. Remarkably, a pancreas-specific Bmal1 knockout results in elevated glucose levels, impaired glucose tolerance, and decreased insulin secretion already at a very young (2–4 months) age [224, 233]. The role of Rev-erbα has also been explored. Glucose-induced insulin secretion and β-cell proliferation are decreased when Rev-erbα is silenced [234]. Moreover, a ß-cell-specific Bmal1 knockout results in hyperglycemia and impaired glucose tolerance [230, 235, 236]. Excitingly, Bmal1 has been suggested to have an antioxidant role since in its absence ROS levels accumulate in the pancreas. In addition, in vitro administration of antioxidant may rescue glucose-induced insulin release via a NRF2-dependent pathway [235]. In summary, these data place the molecular clock as an important regulator of pancreas physiology. In humans, rhythmic clock gene expression has been described [227] and basal and stimulated insulin secretion is dependent on CLOCK [237]. Pancreatic islets from type-2 diabetic patients shown decreased clock gene expression rhythms which are correlated with insulin content and glycated hemoglobin [238]. For an in-depth view of the role of the molecular clock in the pancreas the reader is referred to excellent reviews [239, 240].
In addition to its canonical function as a nutrient absorptive organ, the intestinal tract plays a systemic regulating role due to its interaction the gut microbiome. An increasing body of evidence shows that gut microbiota influences immune responses, intestinal homeostasis, nutrient processing, and pathogen resistance. Many fecal bacteria show rhythms of abundance in the gut. In the absence of Bmal1, these rhythms are largely lost in both genders, but sex-related differences were also reported [241]. Not surprisingly, microbiome disbalance has been associated with increased risks of developing obesity, inflammatory diseases, cancer, diabetes, and psychiatric, respiratory, and metabolic disorders (reviewed in [242, 243]. Concerning intestinal physiological processes, several important parameters such as gastrointestinal motility and emptying [244,245,246], epithelial cell renewal [247,248,249], and absorption of nutrient and electrolytes display oscillatory profiles [250,251,252]. The presence of a local molecular clock has been confirmed in several parts of the intestine in mice and humans [252,253,254]. Strikingly, circadian Nfil3 expression is affected by microbiota, STAT3 (signal transducer and activator of transcription 3), and the epithelial cell circadian clock. Remarkably, NFIL3 affects lipid uptake and storage, and modulates lipid absorption and export, thus placing NFIL3 as an important link between microbiota, molecular clocks, and host metabolism, and therefore, as a putative therapeutic target [255]. Gene knockout models have demonstrated the importance of the circadian clock in intestinal physiology. Clock knockout leads to the absence of circadian patterns of enterocyte activity and loss of food-induced synchronization [252, 256]. Interestingly, rhythmic calcium serum levels have been reported in mice and are lost in mice with targeted Bmal1 deletion in the intestines due to disrupted calcium absorption, which consequently leads to increased calcium bone absorption. This process is dependent on a vitamin D receptor-related pathway [257]. For in-depth descriptions about the role of the molecular clock in intestinal regulation, please refer to recent reviews [250, 258].
The liver is the largest metabolic organ and responsible for a myriad of survival-limiting biological processes, of which we here highlight metabolism of nutrients and bile acids, xenobiotic metabolization, and synthesis of plasma proteins and cholesterol. As an object of study in the early years of molecular chronobiology of peripheral tissues [94], the liver has been one of the best-studied tissues in the circadian field. Reports on circadian oscillations in physiological aspects of the liver date back to the early 1960’s when rhythmic enzyme activity of pyruvate kinase, fatty acid synthetase, and glycogen-related enzymes were first described [259,260,261]. Data from the beginning of the century using circadian microarray analyses estimated that about 10% of all genes are rhythmically expressed in liver [262,263,264]. Recent transcriptomic techniques such as nascent RNA-seq, ChIP-seq, and proteomics, however, report a lack of overlap between rhythmic targets evaluated by each technique when compared to transcriptomics [28, 138, 140, 146], thus implicating post-translational modifications as an important circadian regulatory process Furthermore, circadian oscillations in gene programs associated with metabolism of carbohydrate, lipids, amino acids and bile acid, detoxification, and synthesis of plasma proteins and cholesterol have been documented [36, 262, 263, 265]. Experimental data from global or liver-specific clock gene deletion mice have revealed an important regulatory role of this machinery in both local (hepatocyte) but also systemic homeostasis, as described below. Cry1 overexpression in leptin receptor mutant (db/db) mice was shown to improve insulin sensitivity [24]. Clock knockout mice show reduced plasma levels of insulin and increased circulating cholesterol, glucose, leptin, and triglycerides levels compared to wild-type controls, and consequently, are more prone to develop metabolic syndrome under a HFD regime [224, 266]. Moreover, global Clock knockout mice show a deregulated metabolic profile mainly related to pyrimidine salvage, lipid metabolism, and Krebs cycle-related pathways [267].
Liver-targeted Bmal1 deletion in the liver leads to arrhythmic gene expression of glucose-related genes, increased glucose clearance, and hypoglycemia restricted to the fasting phase [268]. As expected, such responses do not perfectly correlate with the findings of global Bmal1 knockouts in glucose metabolism, but corroborates the regulatory role of the molecular clock in the physiology of the liver [269]. Dual Rev-erbα/β knockout specifically in the liver results in loss of rhythmicity in almost of 90% of wild-type rhythmic transcripts, altered locomotor activity, increased circulating glucose and triglyceride levels, and a reduction in FFAs compared to controls [270]. Such deleterious effects of dual liver-specific gene knockout have been supported by an independent study [271]. Targeted Rev-erbα knockout in the embryonic stage or in adults, on the contrary, leads to only modest alterations in liver physiology [270, 272]. Recently, Rev-erbα/β knockout in the liver was shown to disrupt transcriptional and de-novo lipogenesis rhythms. Interestingly, hepatocyte-targeted deletion of Rev-erbα/β also affects the transcriptome and metabolome of non-hepatocyte cells within the liver. Moreover, in livers of control mice, submitted to time-restricted food access, transcriptomics reveal a phase shift of 12 h in gene expression, which is completely lost in Rev-erbα/β knockouts [273]. On the other hand, liver-specific Rorγ knockout mice show daytime improved insulin sensitivity and glucose responses as result of decreased hepatic gluconeogenesis. In addition, bioinformatic analyses revealed that RORγ is a regulatory player of several glucose-related genes by interacting with ROREs in their promoter regions [274]. In another study, liver-specific Rorγ knockout mice show reduced lipid levels in liver and blood as well as reduced bile acid synthesis [275]. In contrast of the beneficial effects of Rorγ, as described above, the removal of Rorα in the liver results in increased HFD-induced hepatic steatosis through AMPK and liver X receptor α (LXRα)-dependent pathways [276]. Remarkably, circadian lipidomic approaches demonstrate that in absence of Per1 and Per2, a similar fraction (~ 20%) of all lipids continue to display an oscillation. However, lipid composition and phasing are altered in Per1/2 knockout mice fed ad libitum. Such findings demonstrate that, even in the absence of a functional transcriptional circadian clock mechanism, rhythms in lipids may still persist [277]. Food restriction regimes protect against the development of metabolic syndrome in mice [278] in a clock-dependent way [279] as well as in a functional peripheral clock network only in LD, but in a SCN-independent manner [280], thus following the federated model of circadian regulation (See Sect. 1.2). Recent evidence supports an interesting regulatory role of neutrophils in liver rhythms. Reduced neutrophil infiltration, under different experimental conditions, is protective against jetlag and diet-induced liver steatosis in a FGF21-dependent manner [281]. Sirtuin1 (SIRT1) is an important nutrient-sensing protein that interacts with the molecular clock. It upregulates the expression of Nicotinamide phosphoribosyltransferase (Nampt) via E-box interaction. Interestingly, NAMPT is responsible for daily oscillations in intracellular NAD+ levels, which are used by SIRT1 as a cofactor to deacetylate clock- and energy metabolism-related proteins [282, 283]. BMAL1/CLOCK heterodimers bind to Sirt1 promoter leading to its transcription [284]. SIRT1-dependent deacetylation contributes to the rhythmic expression of its target genes to modulate liver physiological processes [282,283,284,285]. SIRT7 deacetylates CRY1, leading to its degradation, as well as regulating liver molecular clock and glucose homeostasis [286].
An important feature of liver is its metabolism of xenobiotics. It has been shown that lipophilic drugs are more rapidly absorbed in the morning compared to the evening in humans while no time-of-day dependence in hydrophilic drug absorption has been reported (reviewed in [287]). Higher morning gene expression of some organic anion transporting polypeptides (OATPs), organic anion transporters (OATs), and organic cation transporters (OCTs) compared to the evening was reported in mice [288]. Phase-I xenobiotic metabolism mainly consists of oxidation, reduction, and hydrolysis, processes that are mainly carried out by cytochrome p450 enzymes. Oscillation at the mRNA levels in several members of the p450 superfamily has been reported with peaks during the dark phase [288]. Interestingly, loss of positive transcriptional factors (Bmal1 or Clock) or negative members (Pers) of the clock TTFL leads to low or high xenobiotic metabolism, respectively [289]. In phase II, detoxification takes place via conjugation to charged compounds such as glutathione, sulfate, and others, thus making a compound more polar and easier to be transported. Gene expression of phase-II-related enzymes has been reported to be abundant during different times within the light phase (peak in the early day or around the day-night transition) [288]. Lastly, phase III comprises several types of membrane transporters of the multidrug resistant protein (MRP) family. Circadian rhythms in this class are less marked, but some transporters show an oscillatory profile, peaking during the light phase in a similar fashion as phase-II genes [288]. Overall, it is accepted that the abundance of phase-I enzyme-related transcripts increases during the dark phase while phase-II and -III transcripts are more abundant during the light phase [288]. More in-depth reviews are found here [290, 291].
Conclusions
The circadian clock system is a complex and pervasive network that directly or indirectly controls a significant portion of all biological processes. Its role as a systemic regulator is seen in experimental models of gene silencing or knockout. Advancements in tissue-specific clock gene modulation has been important for a better understanding of tissue clock gene organization at both cellular as well as systemic levels. In addition to its molecular basis, the organizational aspects of the circadian clock network have received increased attention over the last years. Understanding how this biological timing machinery works at all levels of organization will be important to fully assess the consequences of circadian rhythm disruption by modern lifestyles and devise ways to prevent chronodisruption and treat associated diseases and conditions.
References
Gerhart-Hines Z, Lazar MA (2015) Circadian metabolism in the light of evolution. Endocr Rev 36(3):289–304. https://doi.org/10.1210/er.2015-1007
West AC, Bechtold DA (2015) The cost of circadian desynchrony: evidence, insights and open questions. BioEssays 37(7):777–788. https://doi.org/10.1002/bies.201400173
Ralph MR, Foster RG, Davis FC, Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247(4945):975–978. https://doi.org/10.1126/science.2305266
Smith JG, Sassone-Corsi P (2020) Clock-in, clock-out: circadian timekeeping between tissues. Biochemist 42(2):6–10. https://doi.org/10.1042/bio04202007
Harder L, Oster H (2020) The tissue clock network: driver and gatekeeper of circadian physiology: circadian rhythms are integrated outputs of central and peripheral tissue clocks interacting in a complex manner—from drivers to gatekeepers. BioEssays 42(5):e1900158. https://doi.org/10.1002/bies.201900158
Astiz M, Heyde I, Oster H (2019) Mechanisms of communication in the mammalian circadian timing system. Int J Mol Sci. https://doi.org/10.3390/ijms20020343
Pilorz V, Helfrich-Forster C, Oster H (2018) The role of the circadian clock system in physiology. Pflugers Arch 470(2):227–239. https://doi.org/10.1007/s00424-017-2103-y
Buijs FN, Leon-Mercado L, Guzman-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, Buijs RM (2016) The circadian system: a regulatory feedback network of periphery and brain. Physiology 31(3):170–181. https://doi.org/10.1152/physiol.00037.2015
Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74(2):246–260. https://doi.org/10.1016/j.neuron.2012.04.006
Rijo-Ferreira F, Takahashi JS (2019) Genomics of circadian rhythms in health and disease. Genome Med 11(1):82. https://doi.org/10.1186/s13073-019-0704-0
Roenneberg T, Merrow M (2016) The circadian clock and human health. Curr Biol 26(10):R432-443. https://doi.org/10.1016/j.cub.2016.04.011
Provencio I, Rollag MD, Castrucci AM (2002) Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 415(6871):493. https://doi.org/10.1038/415493a
Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD (1998) Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA 95(1):340–345. https://doi.org/10.1073/pnas.95.1.340
Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA (2002) Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298(5601):2213–2216. https://doi.org/10.1126/science.1076848
Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB (2003) Melanopsin is required for non-image-forming photic responses in blind mice. Science 301(5632):525–527. https://doi.org/10.1126/science.1086179
Foster RG, Hughes S, Peirson SN (2020) Circadian photoentrainment in mice and humans. Biology. https://doi.org/10.3390/biology9070180
Hughes S, Hankins MW, Foster RG, Peirson SN (2012) Melanopsin phototransduction: slowly emerging from the dark. Prog Brain Res 199:19–40. https://doi.org/10.1016/B978-0-444-59427-3.00002-2
Hughes S, Jagannath A, Rodgers J, Hankins MW, Peirson SN, Foster RG (2016) Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye 30(2):247–254. https://doi.org/10.1038/eye.2015.264
Ksendzovsky A, Pomeraniec IJ, Zaghloul KA, Provencio JJ, Provencio I (2017) Clinical implications of the melanopsin-based non-image-forming visual system. Neurology 88(13):1282–1290. https://doi.org/10.1212/WNL.0000000000003761
Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90(3):1063–1102. https://doi.org/10.1152/physrev.00009.2009
Wen S, Ma D, Zhao M, Xie L, Wu Q, Gou L, Zhu C, Fan Y, Wang H, Yan J (2020) Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat Neurosci 23(3):456–467. https://doi.org/10.1038/s41593-020-0586-x
Pilorz V, Astiz M, Heinen KO, Rawashdeh O, Oster H (2020) The concept of coupling in the mammalian circadian clock network. J Mol Biol 432(12):3618–3638. https://doi.org/10.1016/j.jmb.2019.12.037
Sassone-Corsi P (2016) The epigenetic and metabolic language of the circadian clock. In: Sassone-Corsi P, Christen Y (eds) A time for metabolism and hormones. Springer International Publishing, Cham, pp 1–11. https://doi.org/10.1007/978-3-319-27069-2_1
Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111(45):16219–16224. https://doi.org/10.1073/pnas.1408886111
Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18(3):164–179. https://doi.org/10.1038/nrg.2016.150
Papazyan R, Zhang Y, Lazar MA (2016) Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat Struct Mol Biol 23(12):1045–1052. https://doi.org/10.1038/nsmb.3324
Brown SA, Azzi A (2013) Peripheral circadian oscillators in mammals. Handb Exp Pharmacol 217:45–66. https://doi.org/10.1007/978-3-642-25950-0_3
Mauvoisin D (2019) Circadian rhythms and proteomics: it’s all about posttranslational modifications! Wiley Interdiscip Rev Syst Biol Med 11(5):e1450. https://doi.org/10.1002/wsbm.1450
Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H (2013) RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155(4):793–806. https://doi.org/10.1016/j.cell.2013.10.026
Weger M, Diotel N, Dorsemans AC, Dickmeis T, Weger BD (2017) Stem cells and the circadian clock. Dev Biol 431(2):111–123. https://doi.org/10.1016/j.ydbio.2017.09.012
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549. https://doi.org/10.1146/annurev-physiol-021909-135821
Richards J, Gumz ML (2012) Advances in understanding the peripheral circadian clocks. FASEB J 26(9):3602–3613. https://doi.org/10.1096/fj.12-203554
Finger AM, Dibner C, Kramer A (2020) Coupled network of the circadian clocks: a driving force of rhythmic physiology. FEBS Lett 594(17):2734–2769. https://doi.org/10.1002/1873-3468.13898
Tanioka M, Yamada H, Doi M, Bando H, Yamaguchi Y, Nishigori C, Okamura H (2009) Molecular clocks in mouse skin. J Invest Dermatol 129(5):1225–1231. https://doi.org/10.1038/jid.2008.345
Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2(5):297–307. https://doi.org/10.1016/j.cmet.2005.09.009
Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP (2002) Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12(7):540–550. https://doi.org/10.1016/s0960-9822(02)00759-5
Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL (1999) Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140(1):207–218. https://doi.org/10.1210/endo.140.1.6428
Moore RY, Eichler VB (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42(1):201–206. https://doi.org/10.1016/0006-8993(72)90054-6
Husse J, Eichele G, Oster H (2015) Synchronization of the mammalian circadian timing system: light can control peripheral clocks independently of the SCN clock: alternate routes of entrainment optimize the alignment of the body’s circadian clock network with external time. BioEssays 37(10):1119–1128. https://doi.org/10.1002/bies.201500026
Buijs R, Salgado R, Sabath E, Escobar C (2013) Peripheral circadian oscillators: time and food. Prog Mol Biol Transl Sci 119:83–103. https://doi.org/10.1016/b978-0-12-396971-2.00004-x
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14(23):2950–2961. https://doi.org/10.1101/gad.183500
Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6(3):269–278. https://doi.org/10.1046/j.1365-2443.2001.00419.x
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291(5503):490–493. https://doi.org/10.1126/science.291.5503.490
Husse J, Leliavski A, Tsang AH, Oster H, Eichele G (2014) The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J 28(11):4950–4960. https://doi.org/10.1096/fj.14-256594
Husse J, Zhou X, Shostak A, Oster H, Eichele G (2011) Synaptotagmin10-Cre, a driver to disrupt clock genes in the SCN. J Biol Rhythms 26(5):379–389. https://doi.org/10.1177/0748730411415363
Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, Takahashi JS (2014) Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife. https://doi.org/10.7554/eLife.04617
Stevens RG, Zhu Y (2015) Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Philos Trans R Soc Lond B Biol Sci 370(1667):20140120. https://doi.org/10.1098/rstb.2014.0120
Liu L, Keoleian GA, Saitou K (2017) Replacement policy of residential lighting optimized for cost, energy, and greenhouse gas emissions. Environ Res Lett 12(11):114034. https://doi.org/10.1088/1748-9326/aa9447
Blume C, Garbazza C, Spitschan M (2019) Effects of light on human circadian rhythms, sleep and mood. Somnologie 23(3):147–156. https://doi.org/10.1007/s11818-019-00215-x
Tosini G, Ferguson I, Tsubota K (2016) Effects of blue light on the circadian system and eye physiology. Mol Vis 22:61–72
Proykova A, Samaras T, Ion R-M, Bruzell E, Doré J-F, Nicolo M, O’Hagan J, Sánchez-Ramos C, Kerkhof L (2018). Potential Risks Human Health LEDs. https://doi.org/10.13140/RG.2.2.24119.62885
Matheson A, O’Brien L, Reid JA (2014) The impact of shiftwork on health: a literature review. J Clin Nurs 23(23–24):3309–3320. https://doi.org/10.1111/jocn.12524
Figueiro MG, White RD (2013) Health consequences of shift work and implications for structural design. J Perinatol 33(Suppl 1):S17-23. https://doi.org/10.1038/jp.2013.7
IARC (2006) IARC monographs on the evaluation of carcinogenic risks to humans. International agency for research on cancer 98
IARC (2019) IARC Monogr Eval Carcinog Risk Chem Hum. 124: night shift work
Kloog I, Stevens RG, Haim A, Portnov BA (2010) Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21(12):2059–2068. https://doi.org/10.1007/s10552-010-9624-4
Rybnikova NA, Haim A, Portnov BA (2016) Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int J Obes (Lond) 40(5):815–823. https://doi.org/10.1038/ijo.2015.255
Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ (2016) Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev 37(6):584–608. https://doi.org/10.1210/er.2016-1083
Baron KG, Reid KJ (2014) Circadian misalignment and health. Int Rev Psychiatry 26(2):139–154. https://doi.org/10.3109/09540261.2014.911149
Begemann K, Neumann AM, Oster H (2020) Regulation and function of extra-SCN circadian oscillators in the brain. Acta Physiol 229(1):e13446. https://doi.org/10.1111/apha.13446
Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S (2003) Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA 100(11):6795–6800. https://doi.org/10.1073/pnas.0936797100
Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pévet P, Buijs RM (2005) The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci 22(10):2531–2540. https://doi.org/10.1111/j.1460-9568.2005.04439.x
Tahara Y, Takatsu Y, Shiraishi T, Kikuchi Y, Yamazaki M, Motohashi H, Muto A, Sasaki H, Haraguchi A, Kuriki D, Nakamura TJ, Shibata S (2017) Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. NPJ Aging Mech Dis 3:16030. https://doi.org/10.1038/npjamd.2016.30
Dumbell R, Leliavski A, Matveeva O, Blaum C, Tsang AH, Oster H (2016) Dissociation of molecular and endocrine circadian rhythms in male mice lacking bmal1 in the adrenal cortex. Endocrinology 157(11):4222–4233. https://doi.org/10.1210/en.2016-1330
Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G (2006) The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4(2):163–173. https://doi.org/10.1016/j.cmet.2006.07.002
Dickmeis T (2009) Glucocorticoids and the circadian clock. J Endocrinol 200(1):3–22. https://doi.org/10.1677/joe-08-0415
Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T (2005) Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem 280(51):42036–42043. https://doi.org/10.1074/jbc.M509600200
Cipolla-Neto J, Amaral FGD (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr Rev 39(6):990–1028. https://doi.org/10.1210/er.2018-00084
Hardeland R, Madrid JA, Tan DX, Reiter RJ (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res 52(2):139–166. https://doi.org/10.1111/j.1600-079X.2011.00934.x
Russart KLG, Nelson RJ (2018) Light at night as an environmental endocrine disruptor. Physiol Behav 190:82–89. https://doi.org/10.1016/j.physbeh.2017.08.029
Haim A, Zubidat AE (2015) Artificial light at night: melatonin as a mediator between the environment and epigenome. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2014.0121
Sabath E, Salgado-Delgado R, Guerrero-Vargas NN, Guzman-Ruiz MA, del Carmen BM, Escobar C, Buijs RM (2014) Food entrains clock genes but not metabolic genes in the liver of suprachiasmatic nucleus lesioned rats. FEBS Lett 588(17):3104–3110. https://doi.org/10.1016/j.febslet.2014.06.045
Caldelas I, Feillet CA, Dardente H, Eclancher F, Malan A, Gourmelen S, Pévet P, Challet E (2005) Timed hypocaloric feeding and melatonin synchronize the suprachiasmatic clockwork in rats, but with opposite timing of behavioral output. Eur J Neurosci 22(4):921–929. https://doi.org/10.1111/j.1460-9568.2005.04284.x
Mendoza J, Graff C, Dardente H, Pevet P, Challet E (2005) Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci 25(6):1514–1522. https://doi.org/10.1523/JNEUROSCI.4397-04.2005
De Araujo LD, Roa SL, Bueno AC, Coeli-Lacchini FB, Martins CS, Uchoa ET, Antunes-Rodrigues J, Elias LL, Elias PC, Moreira AC, De Castro M (2016) Restricted feeding schedules modulate in a different manner the expression of clock genes in rat hypothalamic nuclei. Front Neurosci 10:567. https://doi.org/10.3389/fnins.2016.00567
Minana-Solis MC, Angeles-Castellanos M, Feillet C, Pevet P, Challet E, Escobar C (2009) Differential effects of a restricted feeding schedule on clock-gene expression in the hypothalamus of the rat. Chronobiol Int 26(5):808–820. https://doi.org/10.1080/07420520903044240
Romanovsky AA (2007) Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292(1):R37-46. https://doi.org/10.1152/ajpregu.00668.2006
Clapham JC (2012) Central control of thermogenesis. Neuropharmacology 63(1):111–123. https://doi.org/10.1016/j.neuropharm.2011.10.014
Liu S, Chen XM, Yoda T, Nagashima K, Fukuda Y, Kanosue K (2002) Involvement of the suprachiasmatic nucleus in body temperature modulation by food deprivation in rats. Brain Res 929(1):26–36. https://doi.org/10.1016/s0006-8993(01)03374-1
Refinetti R, Menaker M (1992) The circadian rhythm of body temperature. Physiol Behav 51(3):613–637. https://doi.org/10.1016/0031-9384(92)90188-8
Refinetti R (1992) Analysis of the circadian rhythm of body temperature. Behav Res Methods 24(1):28–36. https://doi.org/10.3758/bf03203466
Aschoff J (1983) Circadian control of body temperature. J Therm Biol 8(1):143–147. https://doi.org/10.1016/0306-4565(83)90094-3
Moraes MN, de Assis LVM, Magalhaes-Marques KK, Poletini MO, de Lima L, Castrucci AML (2017) Melanopsin, a canonical light receptor, mediates thermal activation of clock genes. Sci Rep 7(1):13977. https://doi.org/10.1038/s41598-017-13939-3
Sporl F, Schellenberg K, Blatt T, Wenck H, Wittern KP, Schrader A, Kramer A (2011) A circadian clock in HaCaT keratinocytes. J Invest Dermatol 131(2):338–348. https://doi.org/10.1038/jid.2010.315
Tamaru T, Hattori M, Honda K, Benjamin I, Ozawa T, Takamatsu K (2011) Synchronization of circadian Per2 rhythms and HSF1-BMAL1:CLOCK interaction in mouse fibroblasts after short-term heat shock pulse. PLoS ONE 6(9):e24521. https://doi.org/10.1371/journal.pone.0024521
Buhr ED, Yoo SH, Takahashi JS (2010) Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330(6002):379–385. https://doi.org/10.1126/science.1195262
Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U (2002) Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 12(18):1574–1583
Poletini MO, Moraes MN, Ramos BC, Jeronimo R, Castrucci AM (2015) TRP channels: a missing bond in the entrainment mechanism of peripheral clocks throughout evolution. Temperature 2(4):522–534. https://doi.org/10.1080/23328940.2015.1115803
Moraes MN, Mezzalira N, de Assis LV, Menaker M, Guler A (1864) Castrucci AM (2017) TRPV1 participates in the activation of clock molecular machinery in the brown adipose tissue in response to light-dark cycle. Biochim Biophys Acta Mol Cell Res 2:324–335. https://doi.org/10.1016/j.bbamcr.2016.11.010
Moraes MN, de Assis LVM, Henriques FDS, Batista ML Jr, Guler AD (1864) Castrucci AML (2017) Cold-sensing TRPM8 channel participates in circadian control of the brown adipose tissue. Biochim Biophys Acta Mol Cell Res 12:2415–2427. https://doi.org/10.1016/j.bbamcr.2017.09.011
Roy D, Levi K, Kiss V, Nevo R, Eisenbach M (2020) Rhodopsin and melanopsin coexist in mammalian sperm cells and activate different signaling pathways for thermotaxis. Sci Rep 10(1):112. https://doi.org/10.1038/s41598-019-56846-5
Perez-Cerezales S, Boryshpolets S, Afanzar O, Brandis A, Nevo R, Kiss V, Eisenbach M (2015) Involvement of opsins in mammalian sperm thermotaxis. Sci Rep 5:16146. https://doi.org/10.1038/srep16146
Segall LA, Milet A, Tronche F, Amir S (2009) Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett 457(1):58–60. https://doi.org/10.1016/j.neulet.2009.03.083
Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289(5488):2344–2347
Ruby NF, Heller HC (1996) Temperature sensitivity of the suprachiasmatic nucleus of ground squirrels and rats in vitro. J Biol Rhythms 11(2):126–136. https://doi.org/10.1177/074873049601100205
Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM (2001) The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology 142(6):2677–2685. https://doi.org/10.1210/endo.142.6.8197
Mendoza J, Lopez-Lopez C, Revel FG, Jeanneau K, Delerue F, Prinssen E, Challet E, Moreau JL, Grundschober C (2011) Dimorphic effects of leptin on the circadian and hypocretinergic systems of mice. J Neuroendocrinol 23(1):28–38. https://doi.org/10.1111/j.1365-2826.2010.02072.x
Grosbellet E, Gourmelen S, Pevet P, Criscuolo F, Challet E (2015) Leptin normalizes photic synchronization in male ob/ob mice, via indirect effects on the suprachiasmatic nucleus. Endocrinology 156(3):1080–1090. https://doi.org/10.1210/en.2014-1570
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762):656–660. https://doi.org/10.1038/45230
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR (2000) The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141(11):4325–4328. https://doi.org/10.1210/endo.141.11.7873
Horvath TL, Abizaid A, Dietrich MO, Li Y, Takahashi JS, Bass J (2012) Ghrelin-immunopositive hypothalamic neurons tie the circadian clock and visual system to the lateral hypothalamic arousal center. Mol Metab 1(1–2):79–85. https://doi.org/10.1016/j.molmet.2012.08.003
Yannielli PC, Molyneux PC, Harrington ME, Golombek DA (2007) Ghrelin effects on the circadian system of mice. J Neurosci 27(11):2890–2895. https://doi.org/10.1523/JNEUROSCI.3913-06.2007
Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA, Rosenbrier-Ribeiro L, Newham P, Clevers H, Bechtold DA, O’Neill JS (2019) Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177(4):896–909. https://doi.org/10.1016/j.cell.2019.02.017
Mendoza J, Pevet P, Challet E (2008) High-fat feeding alters the clock synchronization to light. J Physiol 586(24):5901–5910. https://doi.org/10.1113/jphysiol.2008.159566
Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6(5):414–421. https://doi.org/10.1016/j.cmet.2007.09.006
Omotola O, Legan S, Slade E, Adekunle A, Pendergast JS (2019) Estradiol regulates daily rhythms underlying diet-induced obesity in female mice. Am J Physiol Endocrinol Metab 317(6):E1172–E1181. https://doi.org/10.1152/ajpendo.00365.2019
Palmisano BT, Stafford JM, Pendergast JS (2017) High-fat feeding does not disrupt daily rhythms in female mice because of protection by ovarian hormones. Front Endocrinol 8:44. https://doi.org/10.3389/fendo.2017.00044
Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S (2013) High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci 37(8):1350–1356. https://doi.org/10.1111/ejn.12133
Bookout AL, de Groot MHM, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA (2013) FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19(9):1147–1152. https://doi.org/10.1038/nm.3249
Chavan R, Feillet C, Costa SSF, Delorme JE, Okabe T, Ripperger JA, Albrecht U (2016) Liver-derived ketone bodies are necessary for food anticipation. Nat Commun 7(1):10580. https://doi.org/10.1038/ncomms10580
Agez L, Laurent V, Pevet P, Masson-Pevet M, Gauer F (2007) Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neurosci 144(2):522–530. https://doi.org/10.1016/j.neuroscience.2006.09.030
Gillette MU, McArthur AJ (1996) Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res 73(1–2):135–139. https://doi.org/10.1016/0166-4328(96)00085-x
McArthur AJ, Gillette MU, Prosser RA (1991) Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res 565(1):158–161. https://doi.org/10.1016/0006-8993(91)91748-p
Buijs FN, Cazarez F, Basualdo MC, Scheer FA, Perusquia M, Centurion D, Buijs RM (2014) The suprachiasmatic nucleus is part of a neural feedback circuit adapting blood pressure response. Neurosci 266:197–207. https://doi.org/10.1016/j.neuroscience.2014.02.018
Wolff G, Esser KA (2012) Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sport Exer 44(9):1663–1670. https://doi.org/10.1249/MSS.0b013e318255cf4c
de Assis LVM, Moraes MN, Magalhaes-Marques KK, Kinker GS, da Silveira C-M, Castrucci AML (2018) Non-metastatic cutaneous melanoma induces chronodisruption in central and peripheral circadian clocks. Int J Mol Sci. https://doi.org/10.3390/ijms19041065
Bouabe H, Okkenhaug K (2013) Gene targeting in mice: a review. Methods Mol Biol 1064:315–336. https://doi.org/10.1007/978-1-62703-601-6_23
Haque SN, Booreddy SR, Welsh DK (2019) Effects of BMAL1 manipulation on the brain’s master circadian clock and behavior. Yale J Biol Med 92(2):251–258
Koronowski KB, Kinouchi K, Welz PS, Smith JG, Zinna VM, Shi J, Samad M, Chen S, Magnan CN, Kinchen JM, Li W, Baldi P, Benitah SA, Sassone-Corsi P (2019) Defining the independence of the liver circadian clock. Cell 177(6):1448–1462. https://doi.org/10.1016/j.cell.2019.04.025
Welz PS, Zinna VM, Symeonidi A, Koronowski KB, Kinouchi K, Smith JG, Guillen IM, Castellanos A, Furrow S, Aragon F, Crainiciuc G, Prats N, Caballero JM, Hidalgo A, Sassone-Corsi P, Benitah SA (2019) BMAL1-driven tissue clocks respond independently to light to maintain homeostasis. Cell 178(4):1029. https://doi.org/10.1016/j.cell.2019.07.030
de Assis LV, Moraes MN, da Silveira Cruz-Machado S, Castrucci AM (2016) The effect of white light on normal and malignant murine melanocytes: a link between opsins, clock genes, and melanogenesis. Biochim Biophys Acta 6:1119–1133. https://doi.org/10.1016/j.bbamcr.2016.03.001
de Assis LVM, Moraes MN, Magalhaes-Marques KK, Castrucci AML (2018) Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: unravelling the photosensitive system of the skin. Eur J Cell Biol 97(3):150–162. https://doi.org/10.1016/j.ejcb.2018.01.004
de Assis LVM, Mendes D, Silva MM, Kinker GS, Pereira-Lima I, Moraes MN, Menck CFM (1867) Castrucci AML (2020) Melanopsin mediates UVA-dependent modulation of proliferation, pigmentation, apoptosis, and molecular clock in normal and malignant melanocytes. Biochim Biophys Acta Mol Cell Res 10:118789. https://doi.org/10.1016/j.bbamcr.2020.118789
Buhr ED, Vemaraju S, Diaz N, Lang RA, Van Gelder RN (2019) Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol 29(20):3478–3487. https://doi.org/10.1016/j.cub.2019.08.063
de Assis LVM, Moraes MN, Castrucci AML (2019) The molecular clock in the skin, its functionality, and how it is disrupted in cutaneous melanoma: a new pharmacological target? Cell Mol Life Sci 76(19):3801–3826. https://doi.org/10.1007/s00018-019-03183-5
Ray S, Valekunja UK, Stangherlin A, Howell SA, Snijders AP, Damodaran G, Reddy AB (2020) Circadian rhythms in the absence of the clock gene Bmal1. Science 367(6479):800–806. https://doi.org/10.1126/science.aaw7365
Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, Abbondante S, Tognini P, Orozco-Solis R, Kinouchi K, Wang C, Swerdloff R, Nadeef S, Masri S, Magistretti P, Orlando V, Borrelli E, Uhlenhaut NH, Baldi P, Adamski J, Tschop MH, Eckel-Mahan K, Sassone-Corsi P (2018) Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174(6):1571–1585. https://doi.org/10.1016/j.cell.2018.08.042
Landgraf D, Neumann AM, Oster H (2017) Circadian clock-gastrointestinal peptide interaction in peripheral tissues and the brain. Best Pract Res Clin Endocrinol Metab 31(6):561–571. https://doi.org/10.1016/j.beem.2017.10.007
Landgraf D, Tsang AH, Leliavski A, Koch CE, Barclay JL, Drucker DJ, Oster H (2015) Oxyntomodulin regulates resetting of the liver circadian clock by food. Elife 4:e06253. https://doi.org/10.7554/eLife.06253
Wang J, Symul L, Yeung J, Gobet C, Sobel J, Luck S, Westermark PO, Molina N, Naef F (2018) Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc Natl Acad Sci USA 115(8):E1916–E1925. https://doi.org/10.1073/pnas.1715225115
Chaves I, van der Horst GT, Schellevis R, Nijman RM, Koerkamp MG, Holstege FC, Smidt MP, Hoekman MF (2014) Insulin-FOXO3 signaling modulates circadian rhythms via regulation of clock transcription. Curr Biol 24(11):1248–1255. https://doi.org/10.1016/j.cub.2014.04.018
Dang F, Sun X, Ma X, Wu R, Zhang D, Chen Y, Xu Q, Wu Y, Liu Y (2016) Insulin post-transcriptionally modulates Bmal1 protein to affect the hepatic circadian clock. Nat Commun 7(1):12696. https://doi.org/10.1038/ncomms12696
Sun X, Dang F, Zhang D, Yuan Y, Zhang C, Wu Y, Wang Y, Liu Y (2015) Glucagon-CREB/CRTC2 signaling cascade regulates hepatic BMAL1 protein. J Biol Chem 290(4):2189–2197. https://doi.org/10.1074/jbc.M114.612358
Hill MJ, Suzuki S, Segars JH, Kino T (2016) CRTC2 is a coactivator of GR and couples GR and CREB in the regulation of hepatic gluconeogenesis. Mol Endocrinol 30(1):104–117. https://doi.org/10.1210/me.2015-1237
Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, Gonzalez FJ, Lilley KS, Kyriacou CP, Hastings MH (2007) Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 45(6):1478–1488. https://doi.org/10.1002/hep.21571
Ramanathan C, Kathale ND, Liu D, Lee C, Freeman DA, Hogenesch JB, Cao R, Liu AC (2018) mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet 14(5):e1007369. https://doi.org/10.1371/journal.pgen.1007369
DiTacchio L, Le HD, Vollmers C, Hatori M, Witcher M, Secombe J, Panda S (2011) Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science 333(6051):1881–1885. https://doi.org/10.1126/science.1206022
Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS (2012) Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338(6105):349–354. https://doi.org/10.1126/science.1226339
G Martelot Le D Canella L Symul E Migliavacca F Gilardi R Liechti O Martin K Harshman M Delorenzi B Desvergne W Herr B Deplancke U Schibler J Rougemont N Guex N Hernandez F Naef the Cycli Xc (2012) Genome-wide rna polymerase ii profiles and rna accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles. PLOS Biol 10(11):e1001442. https://doi.org/10.1371/journal.pbio.1001442
Menet JS, Rodriguez J, Abruzzi KC, Rosbash M (2012) Nascent-seq reveals novel features of mouse circadian transcriptional regulation. eLife. https://doi.org/10.7554/eLife.00011
Preussner M, Heyd F (2016) Post-transcriptional control of the mammalian circadian clock: implications for health and disease. Pflugers Arch 468(6):983–991. https://doi.org/10.1007/s00424-016-1820-y
Du NH, Arpat AB, De Matos M, Gatfield D (2014) MicroRNAs shape circadian hepatic gene expression on a transcriptome-wide scale. Elife 3:e02510. https://doi.org/10.7554/eLife.02510
Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K (2007) microRNA modulation of circadian-clock period and entrainment. Neuron 54(5):813–829. https://doi.org/10.1016/j.neuron.2007.05.017
Yoo SH, Kojima S, Shimomura K, Koike N, Buhr ED, Furukawa T, Ko CH, Gloston G, Ayoub C, Nohara K, Reyes BA, Tsuchiya Y, Yoo OJ, Yagita K, Lee C, Chen Z, Yamazaki S, Green CB, Takahashi JS (2017) Period2 3’-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci USA 114(42):E8855-e8864. https://doi.org/10.1073/pnas.1706611114
Robles MS, Humphrey SJ, Mann M (2017) Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab 25(1):118–127. https://doi.org/10.1016/j.cmet.2016.10.004
Ghazalpour A, Bennett B, Petyuk VA, Orozco L, Hagopian R, Mungrue IN, Farber CR, Sinsheimer J, Kang HM, Furlotte N, Park CC, Wen P-Z, Brewer H, Weitz K, Camp DG II, Pan C, Yordanova R, Neuhaus I, Tilford C, Siemers N, Gargalovic P, Eskin E, Kirchgessner T, Smith DJ, Smith RD, Lusis AJ (2011) Comparative analysis of proteome and transcriptome variation in mouse. PLOS Genet 7(6):e1001393. https://doi.org/10.1371/journal.pgen.1001393
Janich P, Arpat AB, Castelo-Szekely V, Lopes M, Gatfield D (2015) Ribosome profiling reveals the rhythmic liver translatome and circadian clock regulation by upstream open reading frames. Genome Res 25(12):1848–1859. https://doi.org/10.1101/gr.195404.115
Jang C, Lahens NF, Hogenesch JB, Sehgal A (2015) Ribosome profiling reveals an important role for translational control in circadian gene expression. Genome Res 25(12):1836–1847. https://doi.org/10.1101/gr.191296.115
Jouffe C, Cretenet G, Symul L, Martin E, Atger F, Naef F, Gachon F (2013) The circadian clock coordinates ribosome biogenesis. PLOS Biol 11(1):e1001455. https://doi.org/10.1371/journal.pbio.1001455
Chiang C-K, Xu B, Mehta N, Mayne J, Sun WYL, Cheng K, Ning Z, Dong J, Zou H, Cheng H-YM, Figeys D (2017) Phosphoproteome profiling reveals circadian clock regulation of posttranslational modifications in the murine hippocampus. Front Neurol 8:110–110. https://doi.org/10.3389/fneur.2017.00110
Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, Li R, Qie J, Zhen B, Wang Y, He F, Qin J, Ding C (2018) A proteomics landscape of circadian clock in mouse liver. Nat Commun 9(1):1553. https://doi.org/10.1038/s41467-018-03898-2
Crosby P, Partch CL (2020) New insights into non-transcriptional regulation of mammalian core clock proteins. J Cell Sci 133(18):241174. https://doi.org/10.1242/jcs.241174
Barnum KJ, O’Connell MJ (2014) Cell cycle regulation by checkpoints. Methods Mol Biol 1170:29–40. https://doi.org/10.1007/978-1-4939-0888-2_2
Yao G (2014) Modelling mammalian cellular quiescence. Interface. Focus 4(3):20130074. https://doi.org/10.1098/rsfs.2013.0074
Tyson JJ, Novak B (2008) Temporal organization of the cell cycle. Curr Biol 18(17):R759–R768. https://doi.org/10.1016/j.cub.2008.07.001
Bieler J, Cannavo R, Gustafson K, Gobet C, Gatfield D, Naef F (2014) Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Mol Syst Biol 10(7):739. https://doi.org/10.15252/msb.20145218
Feillet C, Krusche P, Tamanini F, Janssens RC, Downey MJ, Martin P, Teboul M, Saito S, Lévi FA, Bretschneider T, van der Horst GTJ, Delaunay F, Rand DA (2014) Phase locking and multiple oscillating attractors for the coupled mammalian clock and cell cycle. Proc Natl Acad Sci USA 111(27):9828–9833. https://doi.org/10.1073/pnas.1320474111
Masri S, Cervantes M, Sassone-Corsi P (2013) The circadian clock and cell cycle: interconnected biological circuits. Curr Opin Cell Biol 25(6):730–734. https://doi.org/10.1016/j.ceb.2013.07.013
Feillet C, van der Horst GTJ, Levi F, Rand DA, Delaunay F (2015) Coupling between the circadian clock and cell cycle oscillators: implication for healthy cells and malignant growth. Front Neurol 6:96–96. https://doi.org/10.3389/fneur.2015.00096
Farshadi E, van der Horst GTJ, Chaves I (2020) Molecular links between the circadian clock and the cell cycle. J Mol Biol 432(12):3515–3524. https://doi.org/10.1016/j.jmb.2020.04.003
Davis K, Roden LC, Leaner VD, van der Watt PJ (2019) The tumour suppressing role of the circadian clock. IUBMB Life 71(7):771–780. https://doi.org/10.1002/iub.2005
Shostak A (2017) Circadian clock, cell division, and cancer: from molecules to organism. Int J Mol Sci. https://doi.org/10.3390/ijms18040873
Kettner NM, Katchy CA, Fu L (2014) Circadian gene variants in cancer. Ann Med 46(4):208–220. https://doi.org/10.3109/07853890.2014.914808
Sancar A, Lindsey-Boltz LA, Kang T-H, Reardon JT, Lee JH, Ozturk N (2010) Circadian clock control of the cellular response to DNA damage. FEBS Lett 584(12):2618–2625. https://doi.org/10.1016/j.febslet.2010.03.017
Kumar N, Moreno NC, Feltes BC, Menck CF, Houten BV (2020) Cooperation and interplay between base and nucleotide excision repair pathways: from DNA lesions to proteins. Genet Mol Biol 43(1 suppl. 1):e20190104. https://doi.org/10.1590/1678-4685-gmb-2019-0104
Menck CFM, Munford V (2014) DNA repair diseases: What do they tell us about cancer and aging? Genet Mol Biol 37(1 Suppl):220–233
Kang TH, Reardon JT, Kemp M, Sancar A (2009) Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci USA 106(8):2864–2870. https://doi.org/10.1073/pnas.0812638106
Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A (2010) Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci USA 107(11):4890–4895. https://doi.org/10.1073/pnas.0915085107
Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A (2011) Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA 108(46):18790–18795. https://doi.org/10.1073/pnas.1115249108
Gaddameedhi S, Selby CP, Kemp MG, Ye R, Sancar A (2015) The circadian clock controls sunburn apoptosis and erythema in mouse skin. J Invest Dermatol 135(4):1119–1127. https://doi.org/10.1038/jid.2014.508
Nikkola V, Gronroos M, Huotari-Orava R, Kautiainen H, Ylianttila L, Karppinen T, Partonen T, Snellman E (2018) Circadian time effects on NB-UVB-induced erythema in human skin in vivo. J Invest Dermatol 138(2):464–467. https://doi.org/10.1016/j.jid.2017.08.016
Manzella N, Bracci M, Strafella E, Staffolani S, Ciarapica V, Copertaro A, Rapisarda V, Ledda C, Amati M, Valentino M, Tomasetti M, Stevens RG, Santarelli L (2015) Circadian modulation of 8-oxoguanine DNA damage repair. Sci Rep 5:13752. https://doi.org/10.1038/srep13752
Kim J, Matsunaga N, Koyanagi S, Ohdo S (2009) Clock gene mutation modulates the cellular sensitivity to genotoxic stress through altering the expression of N-methylpurine DNA glycosylase gene. Biochem Pharmacol 78(8):1075–1082. https://doi.org/10.1016/j.bcp.2009.06.013
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772. https://doi.org/10.2147/CIA.S158513
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. https://doi.org/10.2174/157015909787602823
Sani M, Sebai H, Ghanem-Boughanmi N, Boughattas NA, Ben-Attia M (2015) Circadian (about 24-hour) variation in malondialdehyde content and catalase activity of mouse erythrocytes. Redox Rep 20(1):26–32. https://doi.org/10.1179/1351000214y.0000000102
Xu YQ, Zhang D, Jin T, Cai DJ, Wu Q, Lu Y, Liu J, Klaassen CD (2012) Diurnal variation of hepatic antioxidant gene expression in mice. PLoS ONE 7(8):e44237. https://doi.org/10.1371/journal.pone.0044237
Sani M, Sebai H, Gadacha W, Boughattas NA, Reinberg A, Mossadok BA (2006) Catalase activity and rhythmic patterns in mouse brain, kidney and liver. Comp Biochem Physiol B Biochem Mol Biol 145(3–4):331–337. https://doi.org/10.1016/j.cbpb.2006.08.005
Stritesky Larssen K, Lyberg T (2006) Oxidative status–age and circadian variations?—a study in leukocytes/plasma. Neuro Endocrinol Lett 27(4):445–452
Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q (2007) Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res 76(2):269–279. https://doi.org/10.1016/j.cardiores.2007.06.027
Díaz-Muñoz M, Hernández-Muñoz R, Suárez J, Chagoya de Sánchez V (1985) Day-night cycle of lipid peroxidation in rat cerebral cortex and their relationship to the glutathione cycle and superoxide dismutase activity. Neuroscience 16(4):859–863. https://doi.org/10.1016/0306-4522(85)90100-9
Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP (2009) Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging 1(12):979–987. https://doi.org/10.18632/aging.100113
O’Neill JS, Reddy AB (2011) Circadian clocks in human red blood cells. Nature 469(7331):498–503. https://doi.org/10.1038/nature09702
Patel SA, Velingkaar NS, Kondratov RV (2014) Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal 20(18):2997–3006. https://doi.org/10.1089/ars.2013.5671
Wilking M, Ndiaye M, Mukhtar H, Ahmad N (2013) Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal 19(2):192–208. https://doi.org/10.1089/ars.2012.4889
Lafontan M (2008) Advances in adipose tissue metabolism. Int J Obes 32(Suppl 7):S39-51. https://doi.org/10.1038/ijo.2008.237
Shostak A, Meyer-Kovac J, Oster H (2013) Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62(7):2195–2203. https://doi.org/10.2337/db12-1449
Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA (2012) Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med 18(12):1768–1777. https://doi.org/10.1038/nm.2979
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556. https://doi.org/10.1210/jc.2004-0395
Friedrichs M, Kolbe I, Seemann J, Tsang AH, Cherradi L, Klein J, Oster H (2018) Circadian clock rhythms in different adipose tissue model systems. Chronobiol Int 35(11):1543–1552. https://doi.org/10.1080/07420528.2018.1494603
Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55(4):962–970. https://doi.org/10.2337/diabetes.55.04.06.db05-0873
Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A (2005) Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 146(12):5631–5636. https://doi.org/10.1210/en.2005-0771
Otway DT, Mantele S, Bretschneider S, Wright J, Trayhurn P, Skene DJ, Robertson MD, Johnston JD (2011) Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 60(5):1577–1581. https://doi.org/10.2337/db10-1098
Loboda A, Kraft WK, Fine B, Joseph J, Nebozhyn M, Zhang C, He Y, Yang X, Wright C, Morris M, Chalikonda I, Ferguson M, Emilsson V, Leonardson A, Lamb J, Dai H, Schadt E, Greenberg HE, Lum PY (2009) Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomics 2:7. https://doi.org/10.1186/1755-8794-2-7
Wu X, Zvonic S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL, Eckel RH, Mynatt RL, Gimble JM (2007) Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity 15(11):2560–2570. https://doi.org/10.1038/oby.2007.308
Kolbe I, Husse J, Salinas G, Lingner T, Astiz M, Oster H (2016) The SCN clock governs circadian transcription rhythms in murine epididymal white adipose tissue. J Biol Rhythms 31(6):577–587. https://doi.org/10.1177/0748730416666170
Christou S, Wehrens SMT, Isherwood C, Moller-Levet CS, Wu H, Revell VL, Bucca G, Skene DJ, Laing EE, Archer SN, Johnston JD (2019) Circadian regulation in human white adipose tissue revealed by transcriptome and metabolic network analysis. Sci Rep 9(1):2641. https://doi.org/10.1038/s41598-019-39668-3
Stenvers DJ, Jongejan A, Atiqi S, Vreijling JP, Limonard EJ, Endert E, Baas F, Moerland PD, Fliers E, Kalsbeek A, Bisschop PH (2019) Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 62(4):704–716. https://doi.org/10.1007/s00125-019-4813-5
Wang J, Lazar MA (2008) Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol Cell Biol 28(7):2213–2220. https://doi.org/10.1128/MCB.01608-07
Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M (2005) Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 102(34):12071–12076. https://doi.org/10.1073/pnas.0502383102
Duez H, Duhem C, Laitinen S, Patole PS, Abdelkarim M, Bois-Joyeux B, Danan JL, Staels B (2009) Inhibition of adipocyte differentiation by RORalpha. FEBS Lett 583(12):2031–2036. https://doi.org/10.1016/j.febslet.2009.05.019
Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S (2014) PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25(6):293–302. https://doi.org/10.1016/j.tem.2014.04.001
Rosen ED, Hsu C-H, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 16(1):22–26. https://doi.org/10.1101/gad.948702
Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A (2006) High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int 23(5):905–914. https://doi.org/10.1080/07420520600827103
Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS (2019) Exploration and development of PPAR modulators in health and disease: an update of clinical evidence. Int J Mol Sci. https://doi.org/10.3390/ijms20205055
Kiehn J-T, Koch C, Walter M, Brod A, Oster H (2017) Circadian rhythms and clocks in adipose tissues: current insights. Chr Physi Ther 7:7–17. https://doi.org/10.2147/CPT.S116242
Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS (2003) Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab 88(6):2838–2843. https://doi.org/10.1210/jc.2002-021721
Calvani M, Scarfone A, Granato L, Mora EV, Nanni G, Castagneto M, Greco AV, Manco M, Mingrone G (2004) Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes 53(4):939–947. https://doi.org/10.2337/diabetes.53.4.939
Froy O, Garaulet M (2018) The circadian clock in white and brown adipose tissue: mechanistic, endocrine, and clinical aspects. Endocr Rev 39(3):261–273. https://doi.org/10.1210/er.2017-00193
Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17(2):200–205. https://doi.org/10.1038/nm.2297
Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J (2001) Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. Faseb j 15(11):2048–2050. https://doi.org/10.1096/fj.00-0536fje
Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM (2006) Nuclear receptor expression links the circadian clock to metabolism. Cell 126(4):801–810. https://doi.org/10.1016/j.cell.2006.06.050
van den Berg R, Kooijman S, Noordam R, Ramkisoensing A, Abreu-Vieira G, Tambyrajah LL, Dijk W, Ruppert P, Mol IM, Kramar B, Caputo R, Puig LS, de Ruiter EM, Kroon J, Hoekstra M, van der Sluis RJ, Meijer OC, Willems van Dijk K, van Kerkhof LWM, Christodoulides C, Karpe F, Gerhart-Hines Z, Kersten S, Meijer JH, Coomans CP, van Heemst D, Biermasz NR, Rensen PCN (2018) A diurnal rhythm in brown adipose tissue causes rapid clearance and combustion of plasma lipids at wakening. Cell Rep 22(13):3521–3533. https://doi.org/10.1016/j.celrep.2018.03.004
van der Veen DR, Shao J, Chapman S, Leevy WM, Duffield GE (2012) A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity 20(7):1527–1529. https://doi.org/10.1038/oby.2012.78
Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, Pryma DA, Khurana TS, Lazar MA (2013) The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503(7476):410–413. https://doi.org/10.1038/nature12642
Monnier C, Auclair M, Le Cam G, Garcia M-P, Antoine B (2018) The nuclear retinoid-related orphan receptor RORα controls circadian thermogenic programming in white fat depots. Physiol Rep 6(8):e13678–e13678. https://doi.org/10.14814/phy2.13678
Chappuis S, Ripperger JA, Schnell A, Rando G, Jud C, Wahli W, Albrecht U (2013) Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol Metab 2(3):184–193. https://doi.org/10.1016/j.molmet.2013.05.002
Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TC, Lucassen EA, Sips HC, Chatzispyrou IA, Houtkooper RH, Meijer JH, Coomans CP, Biermasz NR, Rensen PC (2015) Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci USA 112(21):6748–6753. https://doi.org/10.1073/pnas.1504239112
Razzoli M, Emmett MJ, Lazar MA, Bartolomucci A (2018) β-Adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. Faseb J 32(10):5640–5646. https://doi.org/10.1096/fj.201800452R
Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM (2003) The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 52(7):1709–1715. https://doi.org/10.2337/diabetes.52.7.1709
La Fleur SE, Kalsbeek A, Wortel J, Buijs RM (1999) A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol 11(8):643–652. https://doi.org/10.1046/j.1365-2826.1999.00373.x
Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466(7306):627–631. https://doi.org/10.1038/nature09253
Mühlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E (2004) Indication of circadian oscillations in the rat pancreas. FEBS Lett 564(1–2):91–96. https://doi.org/10.1016/S0014-5793(04)00322-9
Peschke E, Peschke D (1998) Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia 41(9):1085–1092. https://doi.org/10.1007/s001250051034
Pulimeno P, Mannic T, Sage D, Giovannoni L, Salmon P, Lemeille S, Giry-Laterriere M, Unser M, Bosco D, Bauer C, Morf J, Halban P, Philippe J, Dibner C (2013) Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia 56(3):497–507. https://doi.org/10.1007/s00125-012-2779-7
Ohta Y, Taguchi A, Matsumura T, Nakabayashi H, Akiyama M, Yamamoto K, Fujimoto R, Suetomi R, Yanai A, Shinoda K, Tanizawa Y (2017) Clock gene dysregulation induced by chronic ER stress disrupts beta-cell function. EBioMedicine 18:146–156. https://doi.org/10.1016/j.ebiom.2017.03.040
Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, Schorderet DF, Bonny C (2004) Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol 226(1):59–66. https://doi.org/10.1016/j.mce.2004.06.001
Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J (2015) Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. https://doi.org/10.1126/science.aac4250
Petrenko V, Saini C, Giovannoni L, Gobet C, Sage D, Unser M, Heddad Masson M, Gu G, Bosco D, Gachon F, Philippe J, Dibner C (2017) Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev 31(4):383–398. https://doi.org/10.1101/gad.290379.116
Jacovetti C, Rodriguez-Trejo A, Guay C, Sobel J, Gattesco S, Petrenko V, Saini C, Dibner C, Regazzi R (2017) MicroRNAs modulate core-clock gene expression in pancreatic islets during early postnatal life in rats. Diabetologia 60(10):2011–2020. https://doi.org/10.1007/s00125-017-4348-6
Lee J, Kim MS, Li R, Liu VY, Fu L, Moore DD, Ma K, Yechoor VK (2011) Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets 3(6):381–388. https://doi.org/10.4161/isl.3.6.18157
Vieira E, Marroquí L, Batista TM, Caballero-Garrido E, Carneiro EM, Boschero AC, Nadal A, Quesada I (2012) The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology 153(2):592–601. https://doi.org/10.1210/en.2011-1595
Lee J, Moulik M, Fang Z, Saha P, Zou F, Xu Y, Nelson DL, Ma K, Moore DD, Yechoor VK (2013) Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol 33(11):2327–2338. https://doi.org/10.1128/mcb.01421-12
Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ (2011) An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54(1):120–124. https://doi.org/10.1007/s00125-010-1920-8
Saini C, Petrenko V, Pulimeno P, Giovannoni L, Berney T, Hebrok M, Howald C, Dermitzakis ET, Dibner C (2016) A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes Metab 18(4):355–365. https://doi.org/10.1111/dom.12616
Stamenkovic JA, Olsson AH, Nagorny CL, Malmgren S, Dekker-Nitert M, Ling C, Mulder H (2012) Regulation of core clock genes in human islets. Metabolism 61(7):978–985. https://doi.org/10.1016/j.metabol.2011.11.013
Petrenko V, Philippe J, Dibner C (2018) Time zones of pancreatic islet metabolism. Diabetes Obes Metab 20(Suppl 2):116–126. https://doi.org/10.1111/dom.13383
Vieira E, Burris TP, Quesada I (2014) Clock genes, pancreatic function, and diabetes. Trends Mol Med 20(12):685–693. https://doi.org/10.1016/j.molmed.2014.10.007
Liang X, Bushman FD, FitzGerald GA (2015) Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA 112(33):10479–10484. https://doi.org/10.1073/pnas.1501305112
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19(1):55–71. https://doi.org/10.1038/s41579-020-0433-9
Wu Y, Wan J, Choe U, Pham Q, Schoene NW, He Q, Li B, Yu L, Wang TTY (2019) Interactions between food and gut microbiota: impact on human health. Annu Rev Food Sci Technol 10:389–408. https://doi.org/10.1146/annurev-food-032818-121303
Furukawa Y, Cook IJ, Panagopoulos V, McEvoy RD, Sharp DJ, Simula M (1994) Relationship between sleep patterns and human colonic motor patterns. Gastroenterology 107(5):1372–1381. https://doi.org/10.1016/0016-5085(94)90539-8
Lindberg G, Iwarzon M, Hammarlund B (1996) 24-hour ambulatory electrogastrography in healthy volunteers. Scand J Gastroenterol 31(7):658–664. https://doi.org/10.3109/00365529609009146
Goo RH, Moore JG, Greenberg E, Alazraki NP (1987) Circadian variation in gastric emptying of meals in humans. Gastroenterology 93(3):515–518. https://doi.org/10.1016/0016-5085(87)90913-9
Stokes K, Cooke A, Chang H, Weaver DR, Breault DT, Karpowicz P (2017) The circadian clock gene bmal1 coordinates intestinal regeneration. Cell Mol Gastroenterol Hepatol 4(1):95–114. https://doi.org/10.1016/j.jcmgh.2017.03.011
Karpowicz P, Zhang Y, Hogenesch JB, Emery P, Perrimon N (2013) The circadian clock gates the intestinal stem cell regenerative state. Cell Rep 3(4):996–1004. https://doi.org/10.1016/j.celrep.2013.03.016
Scheving LE, Burns ER, Pauly JE, Tsai TH (1978) Circadian variation in cell division of the mouse alimentary tract, bone marrow and corneal epithelium. Anat Rec 191(4):479–486. https://doi.org/10.1002/ar.1091910407
Pacha J, Sumova A (2013) Circadian regulation of epithelial functions in the intestine. Acta Physiol 208(1):11–24. https://doi.org/10.1111/apha.12090
Pan X, Hussain MM (2007) Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J Biol Chem 282(34):24707–24719. https://doi.org/10.1074/jbc.M701305200
Pan X, Hussain MM (2009) Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res 50(9):1800–1813. https://doi.org/10.1194/jlr.M900085-JLR200
Froy O, Chapnik N (2007) Circadian oscillation of innate immunity components in mouse small intestine. Mol Immunol 44(8):1954–1960. https://doi.org/10.1016/j.molimm.2006.09.026
Pardini L, Kaeffer B, Trubuil A, Bourreille A, Galmiche JP (2005) Human intestinal circadian clock: expression of clock genes in colonocytes lining the crypt. Chronobiol Int 22(6):951–961. https://doi.org/10.1080/07420520500395011
Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper LV (2017) The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 357(6354):912–916. https://doi.org/10.1126/science.aan0677
Pan X, Zhang Y, Wang L, Hussain MM (2010) Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab 12(2):174–186. https://doi.org/10.1016/j.cmet.2010.05.014
Kawai M, Kinoshita S, Yamazaki M, Yamamoto K, Rosen CJ, Shimba S, Ozono K, Michigami T (2019) Intestinal clock system regulates skeletal homeostasis. JCI insight 4(5):e121798. https://doi.org/10.1172/jci.insight.121798
Hussain MM (2014) Regulation of intestinal lipid absorption by clock genes. Annu Rev Nutr 34:357–375. https://doi.org/10.1146/annurev-nutr-071813-105322
Feuers RJ, Casciano DA, Tsai TH, Scheving LE (1987) Regulation of the circadian rhythm of hepatic pyruvate kinase in mice. Prog Clin Biol Res 227:163–172
North C, Feuers RJ, Scheving LE, Pauly JE, Tsai TH, Casciano DA (1981) Circadian organization of thirteen liver and six brain enzymes of the mouse. Am J Anat 162(3):183–199. https://doi.org/10.1002/aja.1001620302
Haus E, Halberg F (1966) Persisting circadian rhythm in hepatic glycogen of mice during inanition and dehydration. Experientia 22(2):113–114. https://doi.org/10.1007/BF01900185
Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417(6884):78–83. https://doi.org/10.1038/nature744
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109(3):307–320. https://doi.org/10.1016/s0092-8674(02)00722-5
Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K-i, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S (2002) A transcription factor response element for gene expression during circadian night. Nature 418(6897):534–539. https://doi.org/10.1038/nature00906
Bass J, Takahashi JS (2010) Circadian integration of metabolism and energetics. Science 330(6009):1349–1354. https://doi.org/10.1126/science.1195027
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308(5724):1043–1045. https://doi.org/10.1126/science.1108750
Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P (2012) Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci USA 109(14):5541–5546. https://doi.org/10.1073/pnas.1118726109
Lamia KA, Storch K-F, Weitz CJ (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105(39):15172–15177. https://doi.org/10.1073/pnas.0806717105
Rudic RD, McNamara P, Curtis A-M, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2(11):e377–e377. https://doi.org/10.1371/journal.pbio.0020377
Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM (2012) Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485(7396):123–127. https://doi.org/10.1038/nature11048
Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA (2012) Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev 26(7):657–667. https://doi.org/10.1101/gad.186858.112
Hunter AL, Pelekanou CE, Adamson A, Downton P, Barron NJ, Cornfield T, Poolman TM, Humphreys N, Cunningham PS, Hodson L, Loudon ASI, Iqbal M, Bechtold DA, Ray DW (2020) Nuclear receptor REVERBα is a state-dependent regulator of liver energy metabolism. Proc Natl Acad Sci USA 117(41):25869–25879. https://doi.org/10.1073/pnas.2005330117
Guan D, Xiong Y, Trinh TM, Xiao Y, Hu W, Jiang C, Dierickx P, Jang C, Rabinowitz JD, Lazar MA (2020) The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 369(6509):1388–1394. https://doi.org/10.1126/science.aba8984
Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM (2014) Retinoic acid-related orphan receptor γ (RORγ): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet 10(5):e1004331. https://doi.org/10.1371/journal.pgen.1004331
Takeda Y, Kang HS, Lih F, Jiang H, Blaner W, Jetten A (2014) Retinoid acid-related orphan receptor, ROR, participates in diurnal transcriptional regulation of lipid metabolic genes. Nucleic Acids Res. https://doi.org/10.1093/nar/gku766
Kim EJ, Yoon YS, Hong S, Son HY, Na TY, Lee MH, Kang HJ, Park J, Cho WJ, Kim SG, Koo SH, Park HG, Lee MO (2012) Retinoic acid receptor-related orphan receptor α-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology 55(5):1379–1388. https://doi.org/10.1002/hep.25529
Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G (2014) Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab 19(2):319–330. https://doi.org/10.1016/j.cmet.2013.12.016
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15(6):848–860. https://doi.org/10.1016/j.cmet.2012.04.019
Patel SA, Velingkaar N, Makwana K, Chaudhari A, Kondratov R (2016) Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci Rep 6(1):25970. https://doi.org/10.1038/srep25970
Kolbe I, Leinweber B, Brandenburger M, Oster H (2019) Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab 30:140–151. https://doi.org/10.1016/j.molmet.2019.09.012
Crespo M, Gonzalez-Teran B, Nikolic I, Mora A, Folgueira C, Rodriguez E, Leiva-Vega L, Pintor-Chocano A, Fernandez-Chacon M, Ruiz-Garrido I, Cicuendez B, Tomas-Loba A, AGCaballero-MolanoBeiroaHernandez-CosidoTorresKennedyDavisBeneditoMarcosNogueirasHidalgoMatesanzLeivaSabio NADLJLNJRJRMRANMG (2020) Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. Elife. https://doi.org/10.7554/eLife.59258
Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G (2016) Histone deacetylase SIRT1 controls proliferation, circadian rhythm, and lipid metabolism during liver regeneration in mice. J Biol Chem 291(44):23318–23329. https://doi.org/10.1074/jbc.M116.737114
Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134(2):317–328. https://doi.org/10.1016/j.cell.2008.06.050
Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q (2014) CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 59(6):2196–2206. https://doi.org/10.1002/hep.26992
Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134(2):329–340. https://doi.org/10.1016/j.cell.2008.07.002
Liu Z, Qian M, Tang X, Hu W, Sun S, Li G, Zhang S, Meng F, Cao X, Sun J, Xu C, Tan B, Pang Q, Zhao B, Wang Z, Guan Y, Ruan X, Liu B (2019) SIRT7 couples light-driven body temperature cues to hepatic circadian phase coherence and gluconeogenesis. Nat Metab 1(11):1141–1156. https://doi.org/10.1038/s42255-019-0136-6
Baraldo M (2008) The influence of circadian rhythms on the kinetics of drugs in humans. Expert Opin Drug Metab Toxicol 4(2):175–192. https://doi.org/10.1517/17425255.4.2.175
Zhang YK, Yeager RL, Klaassen CD (2009) Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 37(1):106–115. https://doi.org/10.1124/dmd.108.024174
DeBruyne JP, Weaver DR, Dallmann R (2014) The hepatic circadian clock modulates xenobiotic metabolism in mice. J Biol Rhythms 29(4):277–287. https://doi.org/10.1177/0748730414544740
Tahara Y, Shibata S (2016) Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat Rev Gastroenterol Hepatol 13(4):217–226. https://doi.org/10.1038/nrgastro.2016.8
Reinke H, Asher G (2016) Circadian clock control of liver metabolic functions. Gastroenterology 150(3):574–580. https://doi.org/10.1053/j.gastro.2015.11.043
Acknowledgements
This work was supported by grants of the German Research Foundation (DFG): HO353-7/1, HO353-10/1, and CRC-296 (TP13). The authors would like to thank Vitor Kennedy from JozuMedia for designing the figures.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Assis, L.V.M., Oster, H. The circadian clock and metabolic homeostasis: entangled networks. Cell. Mol. Life Sci. 78, 4563–4587 (2021). https://doi.org/10.1007/s00018-021-03800-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03800-2