Abstract
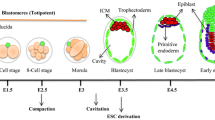
Embryonic stem cells (ESCs) are immortal stem cells that own multi-lineage differentiation potential. ESCs are commonly derived from the inner cell mass (ICM) of pre-implantation embryos. Due to their tremendous developmental capacity and unlimited self-renewal, ESCs have diverse biomedical applications. Different culture media have been developed to procure and maintain ESCs in a state of naïve pluripotency, and to preserve a stable genome and epigenome during serial passaging. Chromatin modifications such as DNA methylation and histone modifications along with microRNA activity and different signaling pathways dynamically contribute to the regulation of the ESC gene regulatory network (GRN). Such modifications undergo remarkable changes in different ESC media and determine the quality and developmental potential of ESCs. In this review, we discuss the current approaches for derivation and maintenance of ESCs, and examine how differences in culture media impact on the characteristics of pluripotency via modulation of GRN during the course of ICM outgrowth into ESCs. We also summarize the current hypotheses concerning the origin of ESCs and provide a perspective about the relationship of these cells to their in vivo counterparts (early embryonic cells around the time of implantation). Finally, we discuss generation of ESCs from human embryos and domesticated animals, and offer suggestions to further advance this fascinating field.






Similar content being viewed by others
References
Pouton CW, Haynes JM (2007) Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov 6:605–616
Abbasalizadeh S, Baharvand H (2013) Technological progress and challenges towards cGMP manufacturing of human pluripotent stem cells based therapeutic products for allogeneic and autologous cell therapies. Biotechnol Adv 31:1600–1623
Tang F, Barbacioru C, Bao S, Lee C, Nordman E, Wang X, Lao K, Surani MA (2010) Tracing the derivation of embryonic stem cells from the inner cell mass by single-cell RNA-Seq analysis. Cell Stem Cell 6:468–478
Totonchi M, Hassani SN, Sharifi-Zarchi A, Tapia N, Adachi K, Arand J, Greber B, Sabour D, Arauzo-Bravo MJ, Walter J, Pakzad M, Gourabi H, Scholer HR, Baharvand H (2017) Blockage of the epithelial-to-mesenchymal transition is required for embryonic stem cell derivation. Stem Cell Rep 9:1275–1290
Boroviak T, Loos R, Bertone P, Smith A, Nichols J (2014) The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat Cell Biol 16:516–528
Hirasawa R, Feil R (2010) Genomic imprinting and human disease. Essays Biochem 48:187–200
Posfai E, Tam OH, Rossant J (2014) Mechanisms of pluripotency in vivo and in vitro. Curr Top Dev Biol 107:1–37
Chazaud C, Yamanaka Y, Pawson T, Rossant J (2006) Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 10:615–624
Yamanaka Y, Lanner F, Rossant J (2010) FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137:715–724
Kojima Y, Kaufman-Francis K, Studdert JB, Steiner KA, Power MD, Loebel DA, Jones V, Hor A, de Alencastro G, Logan GJ, Teber ET, Tam OH, Stutz MD, Alexander IE, Pickett HA, Tam PP (2014) The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell 14:107–120
Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W (2012) The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48:849–862
Yagi M, Yamanaka S, Yamada Y (2017) Epigenetic foundations of pluripotent stem cells that recapitulate in vivo pluripotency. Lab Investig 97:1133–1141
Morgani S, Nichols J, Hadjantonakis AK (2017) The many faces of Pluripotency: in vitro adaptations of a continuum of in vivo states. BMC Dev Biol 17:7
Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, de Sousa Chuva, Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4:487–492
Weinberger L, Ayyash M, Novershtern N, Hanna JH (2016) Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 17:155–169
Hassani SN, Totonchi M, Gourabi H, Scholer HR, Baharvand H (2014) Signaling roadmap modulating naive and primed pluripotency. Stem Cells Dev 23:193–208
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Hassani SN, Pakzad M, Asgari B, Taei A, Baharvand H (2014) Suppression of transforming growth factor beta signaling promotes ground state pluripotency from single blastomeres. Hum Reprod 29:1739–1748
Tada T, Tada M, Hilton K, Barton SC, Sado T, Takagi N, Surani MA (1998) Epigenotype switching of imprintable loci in embryonic germ cells. Dev Genes Evol 207:551–561
Shovlin TC, Durcova-Hills G, Surani A, McLaren A (2008) Heterogeneity in imprinted methylation patterns of pluripotent embryonic germ cells derived from pre-migratory mouse germ cells. Dev Biol 313:674–681
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78:7634–7638
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336:688–690
Ying QL, Nichols J, Chambers I, Smith A (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–292
Batlle-Morera L, Smith A, Nichols J (2008) Parameters influencing derivation of embryonic stem cells from murine embryos. Genesis 46:758–767
Brook FA, Gardner RL (1997) The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci U S A 94:5709–5712
Ptak GE, Tacconi E, Czernik M, Toschi P, Modlinski JA, Loi P (2012) Embryonic diapause is conserved across mammals. PLoS One 7:e33027
Scognamiglio R, Cabezas-Wallscheid N, Thier MC, Altamura S, Reyes A, Prendergast AM, Baumgartner D, Carnevalli LS, Atzberger A, Haas S, von Paleske L, Boroviak T, Worsdorfer P, Essers MA, Kloz U, Eisenman RN, Edenhofer F, Bertone P, Huber W, van der Hoeven F, Smith A, Trumpp A (2016) Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164:668–680
Nichols J, Chambers I, Taga T, Smith A (2001) Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 128:2333–2339
Do DV, Ueda J, Messerschmidt DM, Lorthongpanich C, Zhou Y, Feng B, Guo G, Lin PJ, Hossain MZ, Zhang W, Moh A, Wu Q, Robson P, Ng HH, Poellinger L, Knowles BB, Solter D, Fu XY (2013) A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev 27:1378–1390
Martello G, Smith A (2014) The nature of embryonic stem cells. Annu Rev Cell Dev Biol 30:647–675
Santos J, Pereira CF, Di-Gregorio A, Spruce T, Alder O, Rodriguez T, Azuara V, Merkenschlager M, Fisher AG (2010) Differences in the epigenetic and reprogramming properties of pluripotent and extra-embryonic stem cells implicate chromatin remodelling as an important early event in the developing mouse embryo. Epigenetics Chromatin 3:1
Hermitte S, Chazaud C (2014) Primitive endoderm differentiation: from specification to epithelium formation. Philos Trans R Soc Lond B Biol Sci 369:20130537
Ficz G, Hore TA, Santos F, Lee HJ, Dean W, Arand J, Krueger F, Oxley D, Paul YL, Walter J, Cook SJ, Andrews S, Branco MR, Reik W (2013) FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. Cell Stem Cell 13:351–359
Habibi E, Brinkman AB, Arand J, Kroeze LI, Kerstens HH, Matarese F, Lepikhov K, Gut M, Brun-Heath I, Hubner NC, Benedetti R, Altucci L, Jansen JH, Walter J, Gut IG, Marks H, Stunnenberg HG (2013) Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. Cell Stem Cell 13:360–369
Leitch HG, McEwen KR, Turp A, Encheva V, Carroll T, Grabole N, Mansfield W, Nashun B, Knezovich JG, Smith A, Surani MA, Hajkova P (2013) Naive pluripotency is associated with global DNA hypomethylation. Nat Struct Mol Biol 20:311–316
Buehr M, Smith A (2003) Genesis of embryonic stem cells. Philos Trans R Soc Lond B Biol Sci 358:1397–1402 (discussion 1402)
Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A (2008) The ground state of embryonic stem cell self-renewal. Nature 453:519–523
Ohtsuka S, Niwa H (2015) The differential activation of intracellular signaling pathways confers the permissiveness of embryonic stem cell derivation from different mouse strains. Development 142:431–437
Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A (2008) Capture of authentic embryonic stem cells from rat blastocysts. Cell 135:1287–1298
Wray J, Kalkan T, Smith AG (2010) The ground state of pluripotency. Biochem Soc Trans 38:1027–1032
Martello G, Bertone P, Smith A (2013) Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J 32:2561–2574
Li X, Zhu L, Yang A, Lin J, Tang F, Jin S, Wei Z, Li J, Jin Y (2011) Calcineurin-NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell 8:46–58
Shimizu T, Ueda J, Ho JC, Iwasaki K, Poellinger L, Harada I, Sawada Y (2012) Dual inhibition of Src and GSK3 maintains mouse embryonic stem cells, whose differentiation is mechanically regulated by Src signaling. Stem Cells 30:1394–1404
Dutta D, Ray S, Home P, Larson M, Wolfe MW, Paul S (2011) Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance. Stem Cells 29:618–628
Hassani SN, Totonchi M, Farrokhi A, Taei A, Larijani MR, Gourabi H, Baharvand H (2012) Simultaneous suppression of TGF-beta and ERK signaling contributes to the highly efficient and reproducible generation of mouse embryonic stem cells from previously considered refractory and non-permissive strains. Stem Cell Rev 8:472–481
Hassani SN, Totonchi M, Sharifi-Zarchi A, Mollamohammadi S, Pakzad M, Moradi S, Samadian A, Masoudi N, Mirshahvaladi S, Farrokhi A, Greber B, Arauzo-Bravo MJ, Sabour D, Sadeghi M, Salekdeh GH, Gourabi H, Scholer HR, Baharvand H (2014) Inhibition of TGFbeta signaling promotes ground state pluripotency. Stem Cell Rev 10:16–30
Xu P, Davis RJ (2010) c-Jun NH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal. Mol Cell Biol 30:1329–1340
Tan BS, Kwek J, Wong CK, Saner NJ, Yap C, Felquer F, Morris MB, Gardner DK, Rathjen PD, Rathjen J (2016) Src family kinases and p38 mitogen-activated protein kinases regulate pluripotent cell differentiation in culture. PLoS One 11:e0163244
Choi J, Huebner AJ, Clement K, Walsh RM, Savol A, Lin K, Gu H, Di Stefano B, Brumbaugh J, Kim SY, Sharif J, Rose CM, Mohammad A, Odajima J, Charron J, Shioda T, Gnirke A, Gygi S, Koseki H, Sadreyev RI, Xiao A, Meissner A, Hochedlinger K (2017) Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells. Nature 548:219–223
Yagi M, Kishigami S, Tanaka A, Semi K, Mizutani E, Wakayama S, Wakayama T, Yamamoto T, Yamada Y (2017) Derivation of ground-state female ES cells maintaining gamete-derived DNA methylation. Nature 548:224–227
Yang J, Ryan DJ, Wang W, Tsang JC, Lan G, Masaki H, Gao X, Antunes L, Yu Y, Zhu Z, Wang J, Kolodziejczyk AA, Campos LS, Wang C, Yang F, Zhong Z, Fu B, Eckersley-Maslin MA, Woods M, Tanaka Y, Chen X, Wilkinson AC, Bussell J, White J, Ramirez-Solis R, Reik W, Gottgens B, Teichmann SA, Tam PPL, Nakauchi H, Zou X, Lu L, Liu P (2017) Establishment of mouse expanded potential stem cells. Nature 550:393–397
Menchero S, Rayon T, Andreu MJ, Manzanares M (2017) Signaling pathways in mammalian preimplantation development: linking cellular phenotypes to lineage decisions. Dev Dyn 246:245–261
Zhao H, Jin Y (2017) Signaling networks in the control of pluripotency. Curr Opin Genet Dev 46:141–148
Gomes Fernandes M, Dries R, Roost MS, Semrau S, de Melo Bernardo A, Davis RP, Ramakrishnan R, Szuhai K, Maas E, Umans L, Abon Escalona V, Salvatori D, Deforce D, Van Criekinge W, Huylebroeck D, Mummery C, Zwijsen A, de Sousa Chuva, Lopes SM (2016) BMP-SMAD signaling regulates lineage priming, but is dispensable for self-renewal in mouse embryonic stem cells. Stem Cell Rep 6:85–94
Walter M, Teissandier A, Perez-Palacios R, Bourc’his D (2016) An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in embryonic stem cells. Elife 5:e11418
Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N (2010) Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater 9:82–88
Taleahmad S, Mirzaei M, Samadian A, Hassani SN, Haynes PA, Salekdeh GH, Baharvand H (2017) Low focal adhesion signaling promotes ground state pluripotency of mouse embryonic stem cells. J Proteome Res 16:3585–3595
Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, Abe T, Sato JD, Hata R, Asashima M (2007) Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 25:3005–3015
Hewitson LC, Leese HJ (1993) Energy metabolism of the trophectoderm and inner cell mass of the mouse blastocyst. J Exp Zool 267:337–343
Taleahmad S, Hassani SN, Hosseini Salekdeh G, Baharvand H (2018) Metabolic signature of pluripotent stem cells. Cell J 20:388–395
Taleahmad S, Mirzaei M, Parker LM, Hassani SN, Mollamohammadi S, Sharifi-Zarchi A, Haynes PA, Baharvand H, Salekdeh GH (2015) Proteome analysis of ground state pluripotency. Sci Rep 5:17985
Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J, Beach D (2007) A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal 9:293–299
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21:297–308
Kaelin WG Jr, McKnight SL (2013) Influence of metabolism on epigenetics and disease. Cell 153:56–69
Xu W, Wang F, Yu Z, Xin F (2016) Epigenetics and cellular metabolism. Genet Epigenetics 8:43–51
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122:947–956
Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA (2008) Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22:746–755
Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, Calabrese JM, Dennis LM, Volkert TL, Gupta S, Love J, Hannett N, Sharp PA, Bartel DP, Jaenisch R, Young RA (2008) Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134:521–533
Wang Y, Melton C, Li YP, Shenoy A, Zhang XX, Subramanyam D, Blelloch R (2013) miR-294/miR-302 promotes proliferation, suppresses G1-S restriction point, and inhibits ESC differentiation through separable mechanisms. Cell Rep 4:99–109
Moradi S, Braun T, Baharvand H (2018) miR-302b-3p promotes self-renewal properties in leukemia inhibitory factor-withdrawn embryonic stem cells. Cell J 20:61–72
Melton C, Judson RL, Blelloch R (2010) Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463:621–626
Smith KN, Singh AM, Dalton S (2010) Myc represses primitive endoderm differentiation in pluripotent stem cells. Cell Stem Cell 7:343–354
Judson RL, Babiarz JE, Venere M, Blelloch R (2009) Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol 27:459–461
Li L, Chen K, Wu Y, Long Q, Zhao D, Ma B, Pei D, Liu X (2017) Gadd45a opens up the promoter regions of miR-295 facilitating pluripotency induction. Cell Death Dis 8:e3107
Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Stewart AF, Smith A, Stunnenberg HG (2012) The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149:590–604
Viswanathan SR, Daley GQ, Gregory RI (2008) Selective blockade of microRNA processing by Lin28. Science 320:97–100
Kumar RM, Cahan P, Shalek AK, Satija R, DaleyKeyser A, Li H, Zhang J, Pardee K, Gennert D, Trombetta JJ, Ferrante TC, Regev A, Daley GQ, Collins JJ (2014) Deconstructing transcriptional heterogeneity in pluripotent stem cells. Nature 516:56–61
Moradi S, Sharifi-Zarchi A, Ahmadi A, Mollamohammadi S, Stubenvoll A, Gunther S, Salekdeh GH, Asgari S, Braun T, Baharvand H (2017) Small RNA sequencing reveals Dlk1-Dio3 locus-embedded microRNAs as major drivers of ground-state pluripotency. Stem Cell Rep 9:2081–2096
Yan Y, Yang X, Li TT, Gu KL, Hao J, Zhang Q, Wang Y (2017) Significant differences of function and expression of microRNAs between ground state and serum-cultured pluripotent stem cells. J Genet Genom 44:179–189
Hackett JA, Dietmann S, Murakami K, Down TA, Leitch HG, Surani MA (2013) Synergistic mechanisms of DNA demethylation during transition to ground-state pluripotency. Stem Cell Rep 1:518–531
Yamaji M, Ueda J, Hayashi K, Ohta H, Yabuta Y, Kurimoto K, Nakato R, Yamada Y, Shirahige K, Saitou M (2013) PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell 12:368–382
Sim YJ, Kim MS, Nayfeh A, Yun YJ, Kim SJ, Park KT, Kim CH, Kim KS (2017) 2i maintains a naive ground state in ESCs through two distinct epigenetic mechanisms. Stem Cell Rep 8:1312–1328
Okashita N, Kumaki Y, Ebi K, Nishi M, Okamoto Y, Nakayama M, Hashimoto S, Nakamura T, Sugasawa K, Kojima N, Takada T, Okano M, Seki Y (2014) PRDM14 promotes active DNA demethylation through the ten–eleven translocation (TET)-mediated base excision repair pathway in embryonic stem cells. Development 141:269–280
Okashita N, Sakashita N, Ito K, Mitsuya A, Suwa Y, Seki Y (2015) PRDM14 maintains pluripotency of embryonic stem cells through TET-mediated active DNA demethylation. Biochem Biophys Res Commun 466:138–145
von Meyenn F, Iurlaro M, Habibi E, Liu NQ, Salehzadeh-Yazdi A, Santos F, Petrini E, Milagre I, Yu M, Xie Z, Kroeze LI, Nesterova TB, Jansen JH, Xie H, He C, Reik W, Stunnenberg HG (2016) Impairment of DNA methylation maintenance is the main cause of global demethylation in naive embryonic stem cells. Mol Cell 62:848–861
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326
Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE (2007) Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448:553–560
Joshi O, Wang SY, Kuznetsova T, Atlasi Y, Peng T, Fabre PJ, Habibi E, Shaik J, Saeed S, Handoko L, Richmond T, Spivakov M, Burgess D, Stunnenberg HG (2015) Dynamic reorganization of extremely long-range promoter-promoter interactions between two states of pluripotency. Cell Stem Cell 17:748–757
Boroviak T, Loos R, Lombard P, Okahara J, Behr R, Sasaki E, Nichols J, Smith A, Bertone P (2015) Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev Cell 35:366–382
Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450:1230–1234
Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H (2008) Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135:909–918
Hayashi K, Lopes SM, Tang F, Surani MA (2008) Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3:391–401
Attari F, Sepehri H, Ansari H, Hassani SN, Esfandiari F, Asgari B, Shahverdi A, Baharvand H (2014) Efficient induction of pluripotency in primordial germ cells by dual inhibition of TGF-beta and ERK signaling pathways. Stem Cells Dev 23:1050–1061
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Investig 119:1438–1449
Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA (2010) A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7:51–63
Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL (2010) Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7:64–77
Wang G, Guo X, Hong W, Liu Q, Wei T, Lu C, Gao L, Ye D, Zhou Y, Chen J, Wang J, Wu M, Liu H, Kang J (2013) Critical regulation of miR-200/ZEB2 pathway in Oct4/Sox2-induced mesenchymal-to-epithelial transition and induced pluripotent stem cell generation. Proc Natl Acad Sci USA 110:2858–2863
Moradi S, Asgari S, Baharvand H (2014) Concise review: harmonies played by microRNAs in cell fate reprogramming. Stem Cells 32:3–15
Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D (2011) E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep 12:720–726
An J, Zheng Y, Dann CT (2017) Mesenchymal to epithelial transition mediated by CDH1 promotes spontaneous reprogramming of male germline stem cells to pluripotency. Stem Cell Rep 8:446–459
Shi L, Wu J (2009) Epigenetic regulation in mammalian preimplantation embryo development. Reprod Biol Endocrinol 7:59
Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A (2012) A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484:339–344
Smith ZD, Meissner A (2013) DNA methylation: roles in mammalian development. Nat Rev Genet 14:204–220
Marcho C, Bevilacqua A, Tremblay KD, Mager J (2015) Tissue-specific regulation of Igf2r/Airn imprinting during gastrulation. Epigenetics Chromatin 8:10
Canovas S, Ross PJ (2016) Epigenetics in preimplantation mammalian development. Theriogenology 86:69–79
Mohammed H, Hernando-Herraez I, Savino A, Scialdone A, Macaulay I, Mulas C, Chandra T, Voet T, Dean W, Nichols J, Marioni JC, Reik W (2017) Single-cell landscape of transcriptional heterogeneity and cell fate decisions during mouse early gastrulation. Cell Rep 20:1215–1228
Bibikova M, Laurent LC, Ren B, Loring JF, Fan JB (2008) Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell 2:123–134
Morey L, Santanach A, Blanco E, Aloia L, Nora EP, Bruneau BG, Di Croce L (2015) Polycomb regulates mesoderm cell fate-specification in embryonic stem cells through activation and repression mechanisms. Cell Stem Cell 17:300–315
Migeon BR (2007) Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med 4:97–105
Seller MJ (1987) Neural tube defects and sex ratios. Am J Med Genet 26:699–707
Schulz EG, Meisig J, Nakamura T, Okamoto I, Sieber A, Picard C, Borensztein M, Saitou M, Bluthgen N, Heard E (2014) The two active X chromosomes in female ESCs block exit from the pluripotent state by modulating the ESC signaling network. Cell Stem Cell 14:203–216
Zvetkova I, Apedaile A, Ramsahoye B, Mermoud JE, Crompton LA, John R, Feil R, Brockdorff N (2005) Global hypomethylation of the genome in XX embryonic stem cells. Nat Genet 37:1274–1279
Choi J, Clement K, Huebner AJ, Webster J, Rose CM, Brumbaugh J, Walsh RM, Lee S, Savol A, Etchegaray JP, Gu H, Boyle P, Elling U, Mostoslavsky R, Sadreyev R, Park PJ, Gygi SP, Meissner A, Hochedlinger K (2017) DUSP9 modulates DNA hypomethylation in female mouse pluripotent stem cells. Cell Stem Cell 20(706–719):e7
Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W (2009) Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet 41:488–493
Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, Eckhaus M, Ried T, Bonner WM, Nussenzweig A (2003) H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 114:371–383
Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, Ishibe-Murakami S, Wang B, Tempst P, Hofmann K, Patel DJ, Elledge SJ, Allis CD (2009) WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 457:57–62
Wu T, Liu Y, Wen D, Tseng Z, Tahmasian M, Zhong M, Rafii S, Stadtfeld M, Hochedlinger K, Xiao A (2014) Histone variant H2A.X deposition pattern serves as a functional epigenetic mark for distinguishing the developmental potentials of iPSCs. Cell Stem Cell 15:281–294
Zwaka TP, Thomson JA (2005) A germ cell origin of embryonic stem cells? Development 132:227–233
Chu LF, Surani MA, Jaenisch R, Zwaka TP (2011) Blimp1 expression predicts embryonic stem cell development in vitro. Curr Biol 21:1759–1765
Bao S, Leitch HG, Gillich A, Nichols J, Tang F, Kim S, Lee C, Zwaka T, Li X, Surani MA (2012) The germ cell determinant blimp1 is not required for derivation of pluripotent stem cells. Cell Stem Cell 11:110–117
Ezashi T, Yuan Y, Roberts RM (2016) Pluripotent stem cells from domesticated mammals. Annu Rev Anim Biosci 4:223–253
Chen X, Ye S, Ying QL (2015) Stem cell maintenance by manipulating signaling pathways: past, current and future. BMB Rep 48:668–676
Farzaneh M, Zare M, Hassani SN, Baharvand H (2018) Effects of various culture conditions on pluripotent stem cell derivation from chick embryos. J Cell Biochem 119:6325–6336
Bogliotti YS, Wu J, Vilarino M, Okamura D, Soto DA, Zhong C, Sakurai M, Sampaio RV, Suzuki K, Izpisua Belmonte JC, Ross PJ (2018) Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci USA 115:2090–2095
Wu J, Okamura D, Li M, Suzuki K, Luo C, Ma L, He Y, Li Z, Benner C, Tamura I, Krause MN, Nery JR, Du T, Zhang Z, Hishida T, Takahashi Y, Aizawa E, Kim NY, Lajara J, Guillen P, Campistol JM, Esteban CR, Ross PJ, Saghatelian A, Ren B, Ecker JR, Izpisua Belmonte JC (2015) An alternative pluripotent state confers interspecies chimaeric competency. Nature 521:316–321
Taei A, Hassani SN, Eftekhari-Yazdi P, Rezazadeh Valojerdi M, Nokhbatolfoghahai M, Masoudi NS, Pakzad M, Gourabi H, Baharvand H (2013) Enhanced generation of human embryonic stem cells from single blastomeres of fair and poor-quality cleavage embryos via inhibition of glycogen synthase kinase beta and Rho-associated kinase signaling. Hum Reprod 28:2661–2671
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
O’Leary T, Heindryckx B, Lierman S, van Bruggen D, Goeman JJ, Vandewoestyne M, Deforce D, de Sousa Lopes SM, De Sutter P (2012) Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat Biotechnol 30:278–282
Ware CB (2017) Concise review: lessons from naive human pluripotent cells. Stem Cells 35:35–41
Liu X, Nefzger CM, Rossello FJ, Chen J, Knaupp AS, Firas J, Ford E, Pflueger J, Paynter JM, Chy HS, O’Brien CM, Huang C, Mishra K, Hodgson-Garms M, Jansz N, Williams SM, Blewitt ME, Nilsson SK, Schittenhelm RB, Laslett AL, Lister R, Polo JM (2017) Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nat Methods 14:1055–1062
Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S (2009) Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4:16–19
Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA 107:9222–9227
Buecker C, Chen HH, Polo JM, Daheron L, Bu L, Barakat TS, Okwieka P, Porter A, Gribnau J, Hochedlinger K, Geijsen N (2010) A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell 6:535–546
Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, Campos LS, Rad R, Guo G, Zhang S, Bradley A, Liu P (2011) Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci USA 108:18283–18288
Pomp O, Dreesen O, Leong DF, Meller-Pomp O, Tan TT, Zhou F, Colman A (2011) Unexpected X chromosome skewing during culture and reprogramming of human somatic cells can be alleviated by exogenous telomerase. Cell Stem Cell 9:156–165
Hirano K, Nagata S, Yamaguchi S, Nakagawa M, Okita K, Kotera H, Ainscough J, Tada T (2012) Human and mouse induced pluripotent stem cells are differentially reprogrammed in response to kinase inhibitors. Stem Cells Dev 21:1287–1298
Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D, Flynn P (2014) Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Rep 2:366–381
Ware CB, Wang L, Mecham BH, Shen L, Nelson AM, Bar M, Lamba DA, Dauphin DS, Buckingham B, Askari B, Lim R, Tewari M, Gartler SM, Issa JP, Pavlidis P, Duan Z, Blau CA (2009) Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell 4:359–369
Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S (2010) Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci USA 107:8129–8134
Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, Tesar PJ, Okada J, Margaretha L, Sperber H, Choi M, Blau CA, Treuting PM, Hawkins RD, Cirulli V, Ruohola-Baker H (2014) Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA 111:4484–4489
Smagghe BJ, Stewart AK, Carter MG, Shelton LM, Bernier KJ, Hartman EJ, Calhoun AK, Hatziioannou VM, Lillacci G, Kirk BA, DiNardo BA, Kosik KS, Bamdad C (2013) MUC1* ligand, NM23-H1, is a novel growth factor that maintains human stem cells in a more naive state. PLoS One 8:e58601
Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, Rais Y, Shipony Z, Mukamel Z, Krupalnik V, Zerbib M, Geula S, Caspi I, Schneir D, Shwartz T, Gilad S, Amann-Zalcenstein D, Benjamin S, Amit I, Tanay A, Massarwa R, Novershtern N, Hanna JH (2013) Derivation of novel human ground state naive pluripotent stem cells. Nature 504:282–286
Chan YS, Goke J, Ng JH, Lu X, Gonzales KA, Tan CP, Tng WQ, Hong ZZ, Lim YS, Ng HH (2013) Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 13:663–675
Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, Reik W, Bertone P, Smith A (2014) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158:1254–1269
Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, Lungjangwa T, Imsoonthornruksa S, Stelzer Y, Rangarajan S, D’Alessio A, Zhang J, Gao Q, Dawlaty MM, Young RA, Gray NS, Jaenisch R (2014) Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15:471–487
Duggal G, Warrier S, Ghimire S, Broekaert D, Van der Jeught M, Lierman S, Deroo T, Peelman L, Van Soom A, Cornelissen R, Menten B, Mestdagh P, Vandesompele J, Roost M, Slieker RC, Heijmans BT, Deforce D, De Sutter P, De Sousa Lopes SC, Heindryckx B (2015) Alternative routes to induce naive pluripotency in human embryonic stem cells. Stem Cells 33:2686–2698
Chen H, Aksoy I, Gonnot F, Osteil P, Aubry M, Hamela C, Rognard C, Hochard A, Voisin S, Fontaine E, Mure M, Afanassieff M, Cleroux E, Guibert S, Chen J, Vallot C, Acloque H, Genthon C, Donnadieu C De, Vos J, Sanlaville D, Guerin JF, Weber M, Stanton LW, Rougeulle C, Pain B, Bourillot PY, Savatier P (2015) Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat Commun 6:7095
Qin H, Hejna M, Liu Y, Percharde M, Wossidlo M, Blouin L, Durruthy-Durruthy J, Wong P, Qi Z, Yu J, Qi LS, Sebastiano V, Song JS, Ramalho-Santos M (2016) YAP induces human naive pluripotency. Cell Rep 14:2301–2312
Zimmerlin L, Park TS, Huo JS, Verma K, Pather SR, Talbot CC Jr, Agarwal J, Steppan D, Zhang YW, Considine M, Guo H, Zhong X, Gutierrez C, Cope L, Canto-Soler MV, Friedman AD, Baylin SB, Zambidis ET (2016) Tankyrase inhibition promotes a stable human naive pluripotent state with improved functionality. Development 143:4368–4380
Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, Alagappan R, Frampton GM, Xu P, Muffat J, Santagata S, Powers D, Barrett CB, Young RA, Lee JT, Jaenisch R, Mitalipova M (2010) Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell 141:872–883
Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, Smith A, Nichols J (2016) Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Rep 6:437–446
Acknowledgements
This work was supported by a grant from Royan Institute, the Iranian Council of Stem Cell Research and Technology, the Iran National Science Foundation (INSF), and Iran Science Elites Federation to H.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassani, SN., Moradi, S., Taleahmad, S. et al. Transition of inner cell mass to embryonic stem cells: mechanisms, facts, and hypotheses. Cell. Mol. Life Sci. 76, 873–892 (2019). https://doi.org/10.1007/s00018-018-2965-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2965-y