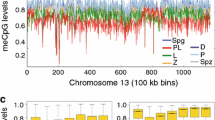
Abstract
De novo germline mutations arise preferentially in male owing to fundamental differences between spermatogenesis and oogenesis. Post-meiotic chromatin remodeling in spermatids results in the elimination of most of the nucleosomal supercoiling and is characterized by transient DNA fragmentation. Using three alternative methods, DNA from sorted populations of mouse spermatids was used to confirm that double-strand breaks (DSB) are created in elongating spermatids and repaired at later steps. Specific capture of DSB was used for whole-genome mapping of DSB hotspots (breakome) for each population of differentiating spermatids. Hotspots are observed preferentially within introns and repeated sequences hence are more prevalent in the Y chromosome. When hotspots arise within genes, those involved in neurodevelopmental pathways become preferentially targeted reaching a high level of significance. Given the non-templated DNA repair in haploid spermatids, transient DSBs formation may, therefore, represent an important component of the male mutation bias and the etiology of neurological disorders, adding to the genetic variation provided by meiosis.






Similar content being viewed by others
References
Iossifov I, Ronemus M, Levy D et al (2012) De novo gene disruptions in children on the autistic spectrum. Neuron 74:285–299. https://doi.org/10.1016/j.neuron.2012.04.009
Kong A, Frigge ML, Masson G et al (2012) Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488:471–475. https://doi.org/10.1038/nature11396
Michaelson JJ, Shi Y, Gujral M et al (2012) Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 151:1431–1442. https://doi.org/10.1016/j.cell.2012.11.019
Neale BM, Kou Y, Liu L et al (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245. https://doi.org/10.1038/nature11011
O’Roak BJ, Vives L, Girirajan S et al (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250. https://doi.org/10.1038/nature10989
Sanders SJ, Murtha MT, Gupta AR et al (2012) De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485:237–241. https://doi.org/10.1038/nature10945
Jiang Y, Yuen RKC, Jin X et al (2013) Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet 93:249–263. https://doi.org/10.1016/j.ajhg.2013.06.012
Zaidi S, Choi M, Wakimoto H et al (2013) De novo mutations in histone-modifying genes in congenital heart disease. Nature 498:220–223. https://doi.org/10.1038/nature12141
Dong S, Walker MF, Carriero NJ et al (2014) De novo insertions and deletions of predominantly paternal origin are associated with autism spectrum disorder. Cell Rep 9:16–23. https://doi.org/10.1016/j.celrep.2014.08.068
Fromer M, Pocklington AJ, Kavanagh DH et al (2014) De novo mutations in schizophrenia implicate synaptic networks. Nature 506:179–184. https://doi.org/10.1038/nature12929
Besenbacher S, Liu S, Izarzugaza JMG et al (2015) Novel variation and de novo mutation rates in population-wide de novo assembled Danish trios. Nat Commun 6:5969. https://doi.org/10.1038/ncomms6969
Francioli LC, Polak PP, Koren A et al (2015) Genome-wide patterns and properties of de novo mutations in humans. Nat Genet 47:822–826. https://doi.org/10.1038/ng.3292
Brandler WM, Antaki D, Gujral M et al (2016) Frequency and complexity of de novo structural mutation in autism. Am J Hum Genet 98:667–679. https://doi.org/10.1016/j.ajhg.2016.02.018
Goldmann JM, Wong WSW, Pinelli M et al (2016) Parent-of-origin-specific signatures of de novo mutations. Nat Genet 48:935–939. https://doi.org/10.1038/ng.3597
Haldane JBS (1947) The mutation rate of the gene for haemophilia, and its segregation ratios in males and females. Ann Eugen 13:262–271
Ellegren H (2007) Characteristics, causes and evolutionary consequences of male-biased mutation. Proc Biol Sci 274:1–10. https://doi.org/10.1098/rspb.2006.3720
Crow JF (2000) The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 1:40–47. https://doi.org/10.1038/35049558
Marcon L, Boissonneault G (2004) Transient DNA strand breaks during mouse and human spermiogenesis new insights in stage specificity and link to chromatin remodeling. Biol Reprod 70:910–918. https://doi.org/10.1095/biolreprod.103.022541
Leduc F, Maquennehan V, Bikond-Nkoma G, Boissonneault G (2008) DNA damage response during chromatin remodeling in elongating spermatids of mice. Biol Reprod 78:324–332. https://doi.org/10.1095/biolreprod.107.064162
Laberge R-M, Boissonneault G (2005) On the nature and origin of DNA strand breaks in elongating spermatids. Biol Reprod 73:289–296. https://doi.org/10.1095/biolreprod.104.036939
Leduc F, Acteau G, Grégoire M-C et al (2011) Post-meiotic DNA damage and response in male germ cells. DNA Repair (Amst). https://doi.org/10.5772/21367
Simard O, Grégoire MC, Arguin M et al (2014) Instability of trinucleotidic repeats during chromatin remodeling in spermatids. Hum Mutat 35:1280–1284. https://doi.org/10.1002/humu.22637
Grégoire M-C, Massonneau J, Leduc F et al (2016) Quantification and genome-wide mapping of DNA double-strand breaks. DNA Repair (Amst) 48:2003. https://doi.org/10.1016/j.dnarep.2016.10.005
Simard O, Leduc F, Acteau G et al (2015) Step-specific sorting of mouse spermatids by flow cytometry. J Vis Exp. https://doi.org/10.3791/53379
Olive PL, Banáth JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1:23–29. https://doi.org/10.1038/nprot.2006.5
TriTek (2003) Autocomet.com : automatic comet assay. http://autocomet.com/index.php. Accessed 11 Apr 2017
Rodrigue S, Materna AC, Timberlake SC et al (2010) Unlocking short read sequencing for metagenomics. PLoS One 5:e11840. https://doi.org/10.1371/journal.pone.0011840
Afgan E, Baker D, van den Beek M et al (2016) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res 44:W3–W10. https://doi.org/10.1093/nar/gkw343
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
(2016) Picard Tools. http://broadinstitute.github.io/picard. Accessed 8 Jul 2016
Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Zhang Y, Liu T, Meyer CA et al (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137. https://doi.org/10.1186/gb-2008-9-9-r137
Erkek S, Hisano M, Liang CY et al (2013) Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol 20:868–875. https://doi.org/10.1038/nsmb.2599
Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. https://doi.org/10.1093/bioinformatics/btq033
Micallef L, Rodgers P (2014) eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. https://doi.org/10.1371/journal.pone.0101717
Gruening BA Galaxy wrapper. https://github.com/bgruening/galaxytools. Accessed 28 Feb 2017
Chen T-W, Li H-P, Lee C-C et al (2014) ChIPseek, a web-based analysis tool for ChIP data. BMC Genom 15:539. https://doi.org/10.1186/1471-2164-15-539
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Tripathi S, Pohl MO, Zhou Y et al (2015) Meta- and orthogonal integration of influenza «OMICs» data defines a role for UBR4 in virus budding. Cell Host Microbe 18:723–735. https://doi.org/10.1016/j.chom.2015.11.002
Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6:1–9. https://doi.org/10.1371/journal.pone.0021800
Ye T, Krebs AR, Choukrallah M-A et al (2011) seqMINER: an integrated ChIP-seq data interpretation platform. Nucleic Acids Res 39:e35. https://doi.org/10.1093/nar/gkq1287
Karolchik D (2004) The UCSC table browser data retrieval tool. Nucleic Acids Res 32:493D–496D. https://doi.org/10.1093/nar/gkh103
Meistrich ML, Reid BO, Barcellona WJ (1976) Changes in sperm nuclei during spermiogenesis and epididymal maturation. Exp Cell Res 99:72–78
Abcam (2017) IHC deparaffinization protocol. http://www.abcam.com/protocols/ihc-deparaffinization-protocol. Accessed 11 Apr 2017
West JA, Viswanathan SR, Yabuuchi A et al (2009) A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460:909–913. https://doi.org/10.1038/nature08210.A
Andres SN, Schellenberg MJ, Wallace BD et al (2015) Recognition and repair of chemically heterogeneous structures at DNA ends. Environ Mol Mutagen 56:1–21. https://doi.org/10.1002/em.21892
Bao W, Kojima KK, Kohany O (2015) Repbase update, a database of repetitive elements in eukaryotic genomes. Mob DNA 6:11. https://doi.org/10.1186/s13100-015-0041-9
Wang D, Zhuang L, Gao B et al (2008) The Blimp-1 gene regulatory region directs EGFP expression in multiple hematopoietic lineages and testis in mice. Transgenic Res 17:193–203. https://doi.org/10.1007/s11248-007-9140-9
Nelms B, Labosky P (2010) PAX genes. In: Transcriptional control of neural crest development. Morgan & Claypool Life Sciences, San Rafael (CA)
Hamaguchi Y, Matsunami N, Yamamoto Y, Honjo T (1989) Purification and characterization of a protein that binds to the recombination signal sequence of the immunoglobulin J kappa segment. Nucleic Acids Res 17:9015–9026
Furukawa T, Maruyama S, Kawaichi M, Honjo T (1992) The Drosophila homolog of the immunoglobulin recombination signal-binding protein regulates peripheral nervous system development. Cell 69:1191–1197
Rathke C, Baarends WM, Jayaramaiah-Raja S et al (2007) Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J Cell Sci 120:1689–1700. https://doi.org/10.1242/jcs.004663
Cabrero J, Palomino-Morales RJ, Camacho JPM (2007) The DNA-repair Ku70 protein is located in the nucleus and tail of elongating spermatids in grasshoppers. Chromosom Res 15:1093–1100. https://doi.org/10.1007/s10577-007-1183-5
Wojtczak A, Popłońska K, Kwiatkowska M (2008) Phosphorylation of H2AX histone as indirect evidence for double-stranded DNA breaks related to the exchange of nuclear proteins and chromatin remodeling in Chara vulgaris spermiogenesis. Protoplasma 233:263–267. https://doi.org/10.1007/s00709-008-0010-y
Gusev O, Suetsugu Y, Cornette R et al (2014) Comparative genome sequencing reveals genomic signature of extreme desiccation tolerance in the anhydrobiotic midge. Nat Commun 5:4784. https://doi.org/10.1038/ncomms5784
Hashimoto T, Horikawa DD, Saito Y et al (2016) Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat Commun 7:12808. https://doi.org/10.1038/ncomms12808
Tang HL, Tang HM, Mak KH et al (2012) Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol Biol Cell 23:2240–2252. https://doi.org/10.1091/mbc.E11-11-0926
Sjakste N, Sjakste T (2007) Possible involvement of DNA strand breaks in regulation of cell differentiation. Eur J Histochem 51:81–94
Koji T, Hishikawa Y (2003) Germ cell apoptosis and its molecular trigger in mouse testes. Arch Histol Cytol 66:1–16
Smith A, Haaf T (1998) DNA nicks and increased sensitivity of DNA to fluorescence in situ end labeling during functional spermiogenesis. Biotechniques 25:496–502
Laiho A, Kotaja N, Gyenesei A, Sironen A (2013) Transcriptome profiling of the murine testis during the first wave of spermatogenesis. PLoS One 8:e61558. https://doi.org/10.1371/journal.pone.0061558
Baudat F, Buard J, Grey C et al (2010) PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327:836–840. https://doi.org/10.1126/science.1183439
Pittoggi C, Renzi L, Zaccagnini G et al (1999) A fraction of mouse sperm chromatin is organized in nucleosomal hypersensitive domains enriched in retroposon DNA. J Cell Sci 112(Pt 2):3537–3548
Shaman JA, Prisztoka R, Ward WS (2006) Topoisomerase IIB and an extracellular nuclease interact to digest sperm DNA in an apoptotic-like manner. Biol Reprod 75:741–748. https://doi.org/10.1095/biolreprod.106.055178
Akematsu T, Fukuda Y, Garg J et al (2017) Post-meiotic DNA double-strand breaks occur in Tetrahymena, and require Topoisomerase II and Spo11. Elife. https://doi.org/10.7554/eLife.26176
Nishibuchi G, Déjardin J (2017) The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosom Res. https://doi.org/10.1007/s10577-016-9547-3
Hurst LD, Ellegren H (1998) Sex biases in the mutation rate. Trends Genet 14:446–452
Pink CJ, Swaminathan SK, Dunham I et al (2009) Evidence that replication-associated mutation alone does not explain between-chromosome differences in substitution rates. Genome Biol Evol 1:13–22. https://doi.org/10.1093/gbe/evp001
Lieber MR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79:181–211. https://doi.org/10.1146/annurev.biochem.052308.093131
Kloosterman WP, Francioli LC, Hormozdiari F et al (2015) Characteristics of de novo structural changes in the human genome. Genome Res 25:792–801. https://doi.org/10.1101/gr.185041.114
Janecka M, Manduca A, Servadio M et al (2015) Effects of advanced paternal age on trajectories of social behavior in offspring. Genes Brain Behav 14:443–453. https://doi.org/10.1111/gbb.12227
Stessman HAF, Xiong B, Coe BP et al (2017) Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet. https://doi.org/10.1038/ng.3792
Yuen RKC, Merico D, Bookman M et al (2017) Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci. https://doi.org/10.1038/nn.4524
Klaus ES, Gonzalez NH, Bergmann M et al (2016) Murine and human spermatids are characterized by numerous, newly synthesized and differentially expressed transcription factors and bromodomain-containing proteins. Biol Reprod 95:1–12. https://doi.org/10.1095/biolreprod.115.137620
Laboratoire de Pierre-Étienne Jacques. http://lab-jacques.recherche.usherbrooke.ca/software_en/vap/home/. Accessed 12 Feb 2017
Acknowledgements
We are grateful to Isabelle Marois for technical assistance with flow cytometry; Julien Massonneau and Olivier Simard for technical help with DBrIC and Sébastien Rodrigue and Dominick Matteau for the preparation of sequencing library. This work was supported by a grant from the Canadian Institutes of Health Research (#MOP-136925) to G.B. and a scholarship from Fonds de recherche du Québec—Santé and the Réseau Québécois en Reproduction to M.-C.G.
Author information
Authors and Affiliations
Contributions
M-CG, FL and GB designed the experiments; M-CG, FL, TC and MA performed the experiments; M-CG, P-EJ and MM performed bioinformatic analyses; MR performed flow cytometry. M-CG and GB wrote the manuscript; all authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grégoire, MC., Leduc, F., Morin, M.H. et al. The DNA double-strand “breakome” of mouse spermatids. Cell. Mol. Life Sci. 75, 2859–2872 (2018). https://doi.org/10.1007/s00018-018-2769-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2769-0