Abstract
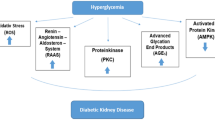
Diabetic kidney disease, a leading cause of end-stage renal disease, has become a serious public health problem worldwide and lacks effective therapies. Autophagy is a highly conserved lysosomal degradation pathway that removes protein aggregates and damaged organelles to maintain cellular homeostasis. As important stress-responsive machinery, autophagy is involved in the pathogenesis of various diseases. Emerging evidence has suggested that dysregulated autophagy may contribute to both glomerular and tubulointerstitial pathologies in kidneys under diabetic conditions. This review summarizes the recent findings regarding the role of autophagy in the pathogenesis of diabetic kidney disease and highlights the regulation of autophagy by the nutrient-sensing pathways and intracellular stress signaling in this disease. The advances in our understanding of autophagy in diabetic kidney disease will facilitate the discovery of a new therapeutic target for the prevention and treatment of this life-threatening diabetes complication.

Similar content being viewed by others
References
USRDS (2003) USRDS: the United States Renal Data System. Am J Kidney Dis 42(6 Suppl 5):1–230
Cao Z, Cooper ME (2011) Pathogenesis of diabetic nephropathy. J Diabetes Investig 2(4):243–247
Shi Y, Hu FB (2014) The global implications of diabetes and cancer. Lancet 383(9933):1947–1948
Rossing P, Hougaard P, Parving HH (2005) Progression of microalbuminuria in type 1 diabetes: ten-year prospective observational study. Kidney Int 68(4):1446–1450
Ahmad J (2015) Management of diabetic nephropathy: recent progress and future perspective. Diabetes Metab Syndr 9(4):343–358
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Noh H, King GL (2007) The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl 106:S49–S53
Calcutt NA et al (2009) Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 8(5):417–429
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070
Kitada M et al (2010) Molecular mechanisms of diabetic vascular complications. J Diabetes Investig 1(3):77–89
Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1):137–188
Brenner BM et al (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345(12):861–869
Lewis EJ et al (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345(12):851–860
Forbes JM, Fukami K, Cooper ME (2007) Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes 115(2):69–84
Ruggenenti P, Cravedi P, Remuzzi G (2010) The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 6(6):319–330
Har R et al (2013) The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia 56(5):1166–1173
Sivitz WI, Yorek MA (2010) Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 12(4):537–577
Sharma K et al (2013) Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24(11):1901–1912
Higgins GC, Coughlan MT (2014) Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol 171(8):1917–1942
Parving HH et al (1981) A prospective study of glomerular filtration rate and arterial blood pressure in insulin-dependent diabetics with diabetic nephropathy. Diabetologia 20(4):457–461
Nath KA (1992) Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20(1):1–17
Burton C, Harris KP (1996) The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis 27(6):765–775
Abbate M, Zoja C, Remuzzi G (2006) How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17(11):2974–2984
American Diabetes A (2009) Standards of medical care in diabetes–2009. Diabetes Care 32(Suppl 1):S13–S61
Yamahara K et al (2013) The role of autophagy in the pathogenesis of diabetic nephropathy. J Diabetes Res 2013:193757
Kume S et al (2014) Autophagy: emerging therapeutic target for diabetic nephropathy. Semin Nephrol 34(1):9–16
Rubinsztein DC, Marino G, Kroemer G (2011) Autophagy and aging. Cell 146(5):682–695
Gonzalez CD et al (2011) The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 7(1):2–11
Ding Y, Choi ME (2015) Autophagy in diabetic nephropathy. J Endocrinol 224(1):R15–R30
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9):814–822
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741
Kuma A, Mizushima N (2010) Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol 21(7):683–690
Mizushima N, Levine B (2010) Autophagy in mammalian development and differentiation. Nat Cell Biol 12(9):823–830
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Choi AM, Ryter SW, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368(7):651–662
Huber TB et al (2012) Emerging role of autophagy in kidney function, diseases and aging. Autophagy 8(7):1009–1031
Axe EL et al (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182(4):685–701
Hayashi-Nishino M et al (2009) A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11(12):1433–1437
Yla-Anttila P et al (2009) 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy 5(8):1180–1185
Hamasaki M et al (2013) Autophagosomes form at ER–mitochondria contact sites. Nature 495(7441):389–393
Lamb CA, Yoshimori T, Tooze SA (2013) The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 14(12):759–774
Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132
Mehrpour M et al (2010) Overview of macroautophagy regulation in mammalian cells. Cell Res 20(7):748–762
Liang C et al (2008) Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 10(7):776–787
Jager S et al (2004) Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117(Pt 20):4837–4848
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293
Maiuri MC et al (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752
Scarlatti F et al (2009) Does autophagy have a license to kill mammalian cells? Cell Death Differ 16(1):12–20
Rubinstein AD, Kimchi A (2012) Life in the balance—a mechanistic view of the crosstalk between autophagy and apoptosis. J Cell Sci 125(Pt 22):5259–5268
Klionsky DJ et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222
Kume S, Thomas MC, Koya D (2012) Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes 61(1):23–29
Barbosa Junior Ade A et al (1992) Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol 61(6):359–366
Han K, Zhou H, Pfeifer U (1997) Inhibition and restimulation by insulin of cellular autophagy in distal tubular cells of the kidney in early diabetic rats. Kidney Blood Pressure Res 20(4):258–263
Vallon V et al (2013) Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304(2):F156–F167
Kitada M et al (2011) Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res 2011:908185
Yamahara K et al (2013) Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol 24(11):1769–1781
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35
Wellen KE, Thompson CB (2010) Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell 40(2):323–332
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89(3):1025–1078
Imai S, Guarente L (2010) Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci 31(5):212–220
Kume S et al (2014) Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int 2014:315494
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122(Pt 20):3589–3594
Inoki K (2014) mTOR signaling in autophagy regulation in the kidney. Semin Nephrol 34(1):2–8
Hosokawa N et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20(7):1981–1991
Jung CH et al (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20(7):1992–2003
Kim J et al (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141
Mori H et al (2009) The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun 384(4):471–475
Zhang MZ et al (2014) Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 63(6):2063–2072
Nagai K et al (2005) Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int 68(2):552–561
Inoki K et al (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Investig 121(6):2181–2196
Godel M et al (2011) Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Investig 121(6):2197–2209
Velagapudi C et al (2011) The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. J Am Soc Nephrol 22(2):262–273
Sakaguchi M et al (2006) Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem Biophys Res Commun 340(1):296–301
Yang Y et al (2007) Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 27(5):495–502
Wittmann S et al (2009) Long-term treatment of sirolimus but not cyclosporine ameliorates diabetic nephropathy in the rat. Transplantation 87(9):1290–1299
Lloberas N et al (2006) Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17(5):1395–1404
Stridh S et al (2015) Inhibition of mTOR activity in diabetes mellitus reduces proteinuria but not renal accumulation of hyaluronan. Upsala J Med Sci 120(4):233–240
Sataranatarajan K et al (2007) Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol 171(6):1733–1742
Fang L et al (2013) Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One 8(4):e60546
Xiao T et al (2014) Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol Cell Biochem 394(1–2):145–154
Kitada M et al (2016) A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia 59(6):1307–1317
Huber TB, Walz G, Kuehn EW (2011) mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 79(5):502–511
Lieberthal W, Levine JS (2009) The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol 20(12):2493–2502
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262
Lee JW et al (2010) The association of AMPK with ULK1 regulates autophagy. PLoS One 5(11):e15394
Alers S et al (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32(1):2–11
Lee MJ et al (2007) A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292(2):F617–F627
Cammisotto PG et al (2008) Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol 294(4):F881–F889
Yamazaki T et al (2009) Combination effects of enalapril and losartan on lipid peroxidation in the kidneys of KK-Ay/Ta mice. Nephron Exp Nephrol 113(2):e66–e76
Ding DF et al (2010) Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am J Nephrol 31(4):363–374
Sokolovska J et al (2010) Influence of metformin on GLUT1 gene and protein expression in rat streptozotocin diabetes mellitus model. Arch Physiol Biochem 116(3):137–145
Kitada M et al (2011) Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 60(2):634–643
Chang CC et al (2011) Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci 18(1):47
Kim MY et al (2013) Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia 56(1):204–217
Kim J et al (2012) Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res 2012:210821
Dugan LL et al (2013) AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Investig 123(11):4888–4899
Zhao L et al (2014) Berberine improves kidney function in diabetic mice via AMPK activation. PLoS One 9(11):e113398
Jin Y et al (2017) Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol 794:106–114
Lee HJ et al (2012) Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J Biol Chem 287(7):4451–4461
Al-Rasheed NM et al (2015) Renoprotective effects of fenofibrate via modulation of LKB1/AMPK mRNA expression and endothelial dysfunction in a rat model of diabetic nephropathy. Pharmacology 95(5–6):229–239
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238
Chang HC, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25(3):138–145
Lee IH et al (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 105(9):3374–3379
Kume S et al (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Investig 120(4):1043–1055
Canto C et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060
Ghosh HS, McBurney M, Robbins PD (2010) SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5(2):e9199
He W et al (2010) Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Investig 120(4):1056–1068
Yacoub R, Lee K, He JC (2014) The role of SIRT1 in diabetic kidney disease. Front Endocrinol (Lausanne) 5:166
Chuang PY et al (2011) Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One 6(8):e23566
Hasegawa K et al (2013) Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 19(11):1496–1504
Shang G et al (2013) 3,5-Diiodo-l-thyronine ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. Biochim Biophys Acta 1832(5):674–684
Li C et al (2010) Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: involvement of SIRT1 and TGF-beta1 pathway. Eur J Pharmacol 649(1–3):382–389
Wu L et al (2012) The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol Biol Rep 39(9):9085–9093
Xu Y et al (2012) Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol 259(3):395–401
Zhang S et al (2012) SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev 133(6):387–400
Ma L et al (2016) Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract 212(4):310–318
Noh H et al (2009) Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol 297(3):F729–F739
Gilbert RE et al (2011) Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int 79(12):1312–1321
Advani A et al (2011) Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol 178(5):2205–2214
Wang X et al (2014) Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int 86(4):712–725
Satriano J, Sharma K (2013) Autophagy and metabolic changes in obesity-related chronic kidney disease. Nephrol Dial Transplant 28(Suppl 4):iv29–iv36
Sohn M et al (2017) Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: the possible role of AMPK autophagy. Am J Physiol Renal Physiol 312(2):F323–F334
Kuwahara S et al (2016) Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol 27(7):1996–2008
Yamamoto T et al (2017) High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J Am Soc Nephrol 28(5):1534–1551
Tagawa A et al (2016) Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes 65(3):755–767
Bondeva T, Wolf G (2014) Reactive oxygen species in diabetic nephropathy: friend or foe? Nephrol Dial Transplant 29(11):1998–2003
Wagener FA et al (2009) The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis 14(12):1451–1458
Koya D et al (2003) Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol 14(8 Suppl 3):S250–S253
Brezniceanu ML et al (2007) Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 71(9):912–923
Tanaka Y et al (2012) Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res 2012:628978
Nishikawa T et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779):787–790
Ma T et al (2013) High glucose induces autophagy in podocytes. Exp Cell Res 319(6):779–789
Yadav A et al (2010) ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol 299(2):C488–C496
Jiang XS et al (2017) Autophagy protects against palmitic acid-induced apoptosis in podocytes in vitro. Sci Rep 7:42764
Liu L et al (2008) Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem 283(45):31153–31162
Sakon S et al (2003) NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22(15):3898–3909
Cunard R (2015) Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med 4(4):715–740
Taniguchi M, Yoshida H (2015) Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hypertens 24(4):345–350
Sieber J et al (2010) Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 299(4):F821–F829
Cao Y et al (2014) Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med 33(4):809–816
Cybulsky AV (2010) Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int 77(3):187–193
Lindenmeyer MT et al (2008) Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19(11):2225–2236
Ohse T et al (2006) Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 70(8):1447–1455
Wu J et al (2010) Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 176(5):2163–2176
Rouschop KM et al (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Investig 120(1):127–141
Ogata M et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231
Chen Y et al (2008) Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol 28(6):1014–1022
Cao A et al (2016) Ursodeoxycholic acid ameliorated diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress. Biol Pharm Bull 39(8):1300–1308
Qi W et al (2011) Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response. Metab Clin Exp 60(5):594–603
Luo ZF et al (2010) Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol Appl Pharmacol 246(1–2):49–57
Cao AL et al (2016) Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Investig 96(6):610–622
Wenger RH (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16(10):1151–1162
Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5(5):343–354
Haase VH (2006) Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291(2):F271–F281
Mazure NM, Pouyssegur J (2010) Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol 22(2):177–180
Rouschop KM, Wouters BG (2009) Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med 9(4):417–424
Tracy K et al (2007) BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 27(17):6229–6242
Zhang H et al (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283(16):10892–10903
Bellot G et al (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29(10):2570–2581
Pagtalunan ME et al (1997) Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Investig 99(2):342–348
Wolf G, Chen S, Ziyadeh FN (2005) From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54(6):1626–1634
Meyer TW, Bennett PH, Nelson RG (1999) Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42(11):1341–1344
Hartleben B et al (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Investig 120(4):1084–1096
Mizushima N et al (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15(3):1101–1111
Sato S et al (2006) Two types of autophagy in the podocytes in renal biopsy specimens: ultrastructural study. J Submicrosc Cytol Pathol 38(2–3):167–174
Chen J et al (2013) mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol 24(2):198–207
Cina DP et al (2012) Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol 23(3):412–420
Oshima Y et al (2011) Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 22(12):2203–2212
Lenoir O et al (2015) Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11(7):1130–1145
Liu J et al (2016) beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis 7:e2183
Sun J et al (2017) Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem Biophys Res Commun 483(1):318–324
Li W et al (2017) FoxO1 promotes mitophagy in the podocytes of diabetic male mice via the PINK1/Parkin pathway. Endocrinology. doi:10.1210/en.2016-1970
Liu S et al (2012) Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8(5):826–837
Kimura T et al (2011) Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22(5):902–913
Livingston MJ, Dong Z (2014) Autophagy in acute kidney injury. Semin Nephrol 34(1):17–26
Havasi A, Dong Z (2016) Autophagy and tubular cell death in the kidney. Semin Nephrol 36(3):174–188
Nair S, Wilding JP (2010) Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 95(1):34–42
Huang C et al (2016) Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci Rep 6:29196
Saito A et al (2005) Significance of proximal tubular metabolism of advanced glycation end products in kidney diseases. Ann N Y Acad Sci 1043:637–643
Liu WJ et al (2015) Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J Biol Chem 290(33):20499–20510
Takahashi A et al (2017) Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 66(5):1359–1372
Fiorentino L et al (2013) Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol Med 5(3):441–455
Xu L et al (2016) Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis 7(11):e2445
Lu X et al (2015) Ursolic acid attenuates diabetic mesangial cell injury through the up-regulation of autophagy via miRNA-21/PTEN/Akt/mTOR suppression. PLoS One 10(2):e0117400
Xavier S et al (2010) BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS One 5(9):e12995
Fan Y et al (2015) BAMBI elimination enhances alternative TGF-beta signaling and glomerular dysfunction in diabetic mice. Diabetes 64(6):2220–2233
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7(12):684–696
Li L et al (2010) Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol 176(4):1767–1778
Forbes MS, Thornhill BA, Chevalier RL (2011) Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: a new look at an old model. Am J Physiol Renal Physiol 301(1):F110–F117
Xu Y et al (2013) Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int J Mol Med 31(3):628–636
Koesters R et al (2010) Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177(2):632–643
Livingston MJ et al (2016) Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12(6):976–998
Baisantry A et al (2016) Autophagy induces prosenescent changes in proximal tubular S3 segments. J Am Soc Nephrol 27(6):1609–1616
Kim WY et al (2012) The role of autophagy in unilateral ureteral obstruction rat model. Nephrology 17(2):148–159
Kim SI et al (2012) Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem 287(15):11677–11688
Ding Y et al (2014) Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol 25(12):2835–2846
Colman RJ et al (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325(5937):201–204
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span—from yeast to humans. Science 328(5976):321–326
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (81528004, 81370791), the National Institutes of Health and Department of Veterans Administration of USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, D., Livingston, M.J., Liu, Z. et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell. Mol. Life Sci. 75, 669–688 (2018). https://doi.org/10.1007/s00018-017-2639-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2639-1