Abstract
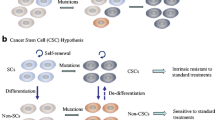
A large body of literature has emerged supporting the importance of cancer stem cells (CSCs) in the pathogenesis of head and neck cancers. CSCs are a subpopulation of cells within a tumor that share the properties of self-renewal and multipotency with stem cells from normal tissue. Their functional relevance to the pathobiology of cancer arises from the unique properties of tumorigenicity, chemotherapy resistance, and their ability to metastasize and invade distant tissues. Several molecular profiles have been used to discriminate a stem cell from a non-stem cell. CSCs can be grown for study and further enriched using a number of in vitro techniques. An evolving option for translational research is the use of mathematical and computational models to describe the role of CSCs in complex tumor environments. This review is focused discussing the evidence emerging from modeling approaches that have clarified the impact of CSCs to the biology of cancer.



Similar content being viewed by others
Abbreviations
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPV:
-
Human papilloma virus
- CSC:
-
Cancer stem cell
- PDX:
-
Patient-derived xenograft
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29. doi:10.3322/caac.21208
Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS, Bray F, Gillison ML (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31(36):4550–4559. doi:10.1200/jco.2013.50.3870
Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, Foote RL, Gilbert J, Gillison ML, Haddad RI, Haughey BH, Hicks WL Jr, Hitchcock YJ, Jimeno A, Kies MS, Lydiatt WM, Maghami E, McCaffrey T, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rodriguez CP, Samant S, Shah JP, Weber RS, Wolf GT, Worden F, Yom SS, McMillian N, Hughes M (2015) Head and neck cancers, version 1.2015. J Natl Compr Cancer Netw 13(7):847–856
Speight PM, Barrett AW (2002) Salivary gland tumours. Oral Dis 8(5):229–240
DeVita VT, Hellman S, Rosenberg SA (2005) Cancer, principles and practice of oncology, 7th edn. Lippincott Williams & Wilkins, Philadelphia
Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R (2004) Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst 96(2):99–106
Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF Jr (1988) Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 48(11):3282–3287
Tuyns AJ, Esteve J, Raymond L, Berrino F, Benhamou E, Blanchet F, Boffetta P, Crosignani P, del Moral A, Lehmann W et al (1988) Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int J Cancer 41(4):483–491
Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14(2):467–475. doi:10.1158/1055-9965.epi-04-0551
Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantu G, Squadrelli M, Quattrone P, Locati LD, Bergamini C, Olmi P, Pierotti MA, Pilotti S (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24(36):5630–5636. doi:10.1200/jco.2005.04.6136
Edge SB, American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York
van Wilgen CP, Dijkstra PU, van der Laan BF, Plukker JT, Roodenburg JL (2004) Morbidity of the neck after head and neck cancer therapy. Head Neck 26(9):785–791. doi:10.1002/hed.20008
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359(11):1116–1127. doi:10.1056/NEJMoa0802656
Tobacco FaDAOoMPa (2006) Erbitux (ceuximab) approved for use in combination with radiation therapy. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm095662.htm
Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (2013) SEER cancer statistics review, 1975–2010. National Cancer Institute. http://seer.cancer.gov/archive/csr/1975_2010/
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349(22):2091–2098. doi:10.1056/NEJMoa031317
Argiris A, Karamouzis MV, Raben D, Ferris RL (2008) Head and neck cancer. Lancet (London, England) 371(9625):1695–1709. doi:10.1016/s0140-6736(08)60728-x
Perez-Ordonez B, Beauchemin M, Jordan RC (2006) Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol 59(5):445–453. doi:10.1136/jcp.2003.007641
Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK (1996) Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med 2(6):682–685
McCaul JA, Gordon KE, Clark LJ, Parkinson EK (2002) Telomerase inhibition and the future management of head-and-neck cancer. Lancet Oncol 3(5):280–288
Grandis JR, Tweardy DJ (1993) Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 53(15):3579–3584
Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ (1998) Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 90(11):824–832
Riedel F, Zaiss I, Herzog D, Gotte K, Naim R, Hormann K (2005) Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res 25(4):2761–2765
Mako V, Czucz J, Weiszhar Z, Herczenik E, Matko J, Prohaszka Z, Cervenak L (2010) Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1beta, TNF-alpha, and LPS. Cytometry A 77(10):962–970. doi:10.1002/cyto.a.20952
Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD (2000) Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA 97(8):4227–4232
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science (New York, NY) 258(5089):1798–1801
Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM (1994) Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 179(5):1409–1415
Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL (1995) Interleukin-8 expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 121(2):202–209
Cancer Genome Atlas Network (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517(7536):576–582. doi:10.1038/nature14129
Huang MF, Lin WL, Ma YC (2005) A study of reactive oxygen species in mainstream of cigarette. Indoor Air 15(2):135–140. doi:10.1111/j.1600-0668.2005.00330.x
Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F (2007) Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 13(5):567–569. doi:10.1038/nm1583
Valavanidis A, Vlachogianni T, Fiotakis K (2009) Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 6(2):445–462. doi:10.3390/ijerph6020445
Churg A, Dai J, Tai H, Xie C, Wright JL (2002) Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med 166(6):849–854. doi:10.1164/rccm.200202-097OC
Chung KF (2005) Inflammatory mediators in chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 4(6):619–625
Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ (2005) HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med 39(3):355–364. doi:10.1016/j.freeradbiomed.2005.03.026
Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R (2008) Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol (Baltimore, MD: 1950) 181(2):1536–1547
Liu X, Togo S, Al-Mugotir M, Kim H, Fang Q, Kobayashi T, Wang X, Mao L, Bitterman P, Rennard S (2008) NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir Res 9:66. doi:10.1186/1465-9921-9-66
Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS (2010) Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol (Baltimore, MD: 1950) 185(5):3035–3040. doi:10.4049/jimmunol.1000252
Chung KY, Mukhopadhyay T, Kim J, Casson A, Ro JY, Goepfert H, Hong WK, Roth JA (1993) Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res 53(7):1676–1683
Chung CH, Gillison ML (2009) Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res 15(22):6758–6762. doi:10.1158/1078-0432.ccr-09-0784
Scheffner M, Huibregtse JM, Vierstra RD, Howley PM (1993) The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75(3):495–505
Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, Lorincz AT, Hedrick L, Cho KR (1993) Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA 90(9):3988–3992
Dyson N, Howley PM, Munger K, Harlow E (1989) The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science (New York, NY) 243(4893):934–937
Huh K, Zhou X, Hayakawa H, Cho JY, Libermann TA, Jin J, Harper JW, Munger K (2007) Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol 81(18):9737–9747. doi:10.1128/jvi.00881-07
Wagers AJ, Weissman IL (2004) Plasticity of adult stem cells. Cell 116(5):639–648. doi:10.1016/S0092-8674(04)00208-9
Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118(5):635–648. doi:10.1016/j.cell.2004.08.012
Prince ME, Ailles LE (2008) Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol 26(17):2871–2875. doi:10.1200/jco.2007.15.1613
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414(6859):105–111. doi:10.1038/35102167
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401. doi:10.1038/nature03128
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65(23):10946–10951. doi:10.1158/0008-5472.can-05-2018
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65(20):9328–9337. doi:10.1158/0008-5472.can-05-1343
Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 104(3):973–978. doi:10.1073/pnas.0610117104
Chen ZG (2009) The cancer stem cell concept in progression of head and neck cancer. J Oncol 2009:894064. doi:10.1155/2009/894064
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355(12):1253–1261. doi:10.1056/NEJMra061808
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM (2006) Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66(19):9339–9344. doi:10.1158/0008-5472.can-06-3126
Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM (1990) A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 345(6277):686–692. doi:10.1038/345686a0
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113(5):643–655
Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17(1):126–140. doi:10.1101/gad.224503
Li M, Pevny L, Lovell-Badge R, Smith A (1998) Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol 8(17):971–974
Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24(4):372–376. doi:10.1038/74199
Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O’Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Ribeiro U Jr, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M (2009) SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 41(11):1238–1242. doi:10.1038/ng.465
Chen SF, Chang YC, Nieh S, Liu CL, Yang CY, Lin YS (2012) Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS One 7(2):e31864. doi:10.1371/journal.pone.0031864
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF (2008) Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res 14(13):4085–4095. doi:10.1158/1078-0432.ccr-07-4404
Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY (2011) Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol 40(8):621–628. doi:10.1111/j.1600-0714.2011.01015.x
Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nor JE (2010) Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res 70(23):9969–9978. doi:10.1158/0008-5472.can-10-1712
Keith B, Simon MC (2007) Hypoxia-inducible factors, stem cells, and cancer. Cell 129(3):465–472. doi:10.1016/j.cell.2007.04.019
McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM (2006) Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 281(34):24171–24181. doi:10.1074/jbc.M604507200
Zhang P, Zhang Y, Mao L, Zhang Z, Chen W (2009) Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett 277(2):227–234. doi:10.1016/j.canlet.2008.12.015
Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME (2010) Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 32(9):1195–1201. doi:10.1002/hed.21315
Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, Ku HH, Chiou SH, Lo WL (2009) Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun 385(3):307–313. doi:10.1016/j.bbrc.2009.05.048
Chikamatsu K, Ishii H, Takahashi G, Okamoto A, Moriyama M, Sakakura K, Masuyama K (2012) Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck 34(3):336–343. doi:10.1002/hed.21732
Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME (2011) Head and neck cancer stem cells: the side population. Laryngoscope 121(3):527–533. doi:10.1002/lary.21032
Sun S, Wang Z (2011) Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer 129(10):2337–2348. doi:10.1002/ijc.25927
Nor C, Zhang Z, Warner KA, Bernardi L, Visioli F, Helman JI, Roesler R, Nor JE (2014) Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia (New York, NY) 16(2):137–146. doi:10.1593/neo.131744
Chen YC, Chang CJ, Hsu HS, Chen YW, Tai LK, Tseng LM, Chiou GY, Chang SC, Kao SY, Chiou SH, Lo WL (2010) Inhibition of tumorigenicity and enhancement of radiochemosensitivity in head and neck squamous cell cancer-derived ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol 46(3):158–165. doi:10.1016/j.oraloncology.2009.11.007
McDermott SC (2015) Elucidating the molecular pathways active in chemo-resistant head and neck cancer stem cells and their role in resistance. University of Michigan Ann Arbor, Michigan
Goldman A, Majumder B, Dhawan A, Ravi S, Goldman D, Kohandel M, Majumder PK, Sengupta S (2015) Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat Commun 6:6139. doi:10.1038/ncomms7139
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414(6859):98–104. doi:10.1038/35102160
Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE (2011) Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One 6(1):e16466. doi:10.1371/journal.pone.0016466
Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D, Myers JN (2010) TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene 29(14):2047–2059. doi:10.1038/onc.2009.486
Yang J, Mani SA, Weinberg RA (2006) Exploring a new twist on tumor metastasis. Cancer Res 66(9):4549–4552. doi:10.1158/0008-5472.can-05-3850
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ (2008) Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 10(3):295–305. doi:10.1038/ncb1691
Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nor JE (2009) Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia (New York, NY) 11(6):583–593
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD (2010) A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett 289(2):151–160. doi:10.1016/j.canlet.2009.08.010
Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, Maccallum J (2009) In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck 31(1):94–101. doi:10.1002/hed.20935
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183(4):1797–1806
Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK (2004) A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA 101(39):14228–14233. doi:10.1073/pnas.0400067101
Yanamoto S, Kawasaki G, Yamada S, Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T, Umeda M (2011) Isolation and characterization of cancer stem-like side population cells in human oral cancer cells. Oral Oncol 47(9):855–860. doi:10.1016/j.oraloncology.2011.06.501
Song J, Chang I, Chen Z, Kang M, Wang CY (2010) Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One 5(7):e11456. doi:10.1371/journal.pone.0011456
Zhang Y, Lu H, Dazin P, Kapila Y (2004) Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin alphav mediate survival signals through focal adhesion kinase. J Biol Chem 279(46):48342–48349. doi:10.1074/jbc.M407953200
Swan EA, Jasser SA, Holsinger FC, Doan D, Bucana C, Myers JN (2003) Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol 39(7):648–655
Kupferman ME, Patel V, Sriuranpong V, Amornphimoltham P, Jasser SA, Mandal M, Zhou G, Wang J, Coombes K, Multani A, Pathak S, Silvio Gutkind J, Myers JN (2007) Molecular analysis of anoikis resistance in oral cavity squamous cell carcinoma. Oral Oncol 43(5):440–454. doi:10.1016/j.oraloncology.2006.04.016
Bunek J, Kamarajan P, Kapila YL (2011) Anoikis mediators in oral squamous cell carcinoma. Oral Dis 17(4):355–361. doi:10.1111/j.1601-0825.2010.01763.x
Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma W, Zhang Y, Li C, Zhang S (2010) Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett 299(2):150–160. doi:10.1016/j.canlet.2010.08.013
Kamarajan P, Alhazzazi TY, Danciu T, D’Silva NJ, Verdin E, Kapila YL (2012) Receptor-interacting protein (RIP) and Sirtuin-3 (SIRT3) are on opposite sides of anoikis and tumorigenesis. Cancer 118(23):5800–5810. doi:10.1002/cncr.27655
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9(5):391–403. doi:10.1016/j.ccr.2006.03.030
Hueng DY, Sytwu HK, Huang SM, Chang C, Ma HI (2011) Isolation and characterization of tumor stem-like cells from human meningiomas. J Neurooncol 104(1):45–53. doi:10.1007/s11060-010-0469-1
Krishnamurthy S, Nor JE (2013) Orosphere assay: a method for propagation of head and neck cancer stem cells. Head Neck 35(7):1015–1021. doi:10.1002/hed.23076
Timmins N, Dietmair S, Nielsen L (2004) Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis 7(2):97–103. doi:10.1007/s10456-004-8911-7
Abaan OD, Polley EC, Davis SR, Zhu YJ, Bilke S, Walker RL, Pineda M, Gindin Y, Jiang Y, Reinhold WC, Holbeck SL, Simon RM, Doroshow JH, Pommier Y, Meltzer PS (2013) The exomes of the NCI-60 panel: a genomic resource for cancer biology and systems pharmacology. Cancer Res 73(14):4372–4382. doi:10.1158/0008-5472.can-12-3342
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA (2001) Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 84(10):1424–1431. doi:10.1054/bjoc.2001.1796
Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, Bradford CR, Carey TE (2010) Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck 32(4):417–426. doi:10.1002/hed.21198
Fiebig HH, Neumann HA, Henss H, Koch H, Kaiser D, Arnold H (1985) Development of three human small cell lung cancer models in nude mice. Recent results in cancer research. Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 97:77–86
Braakhuis BJ, Sneeuwloper G, Snow GB (1984) The potential of the nude mouse xenograft model for the study of head and neck cancer. Arch Otorhinolaryngol 239(1):69–79
Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A (2014) Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4(9):998–1013. doi:10.1158/2159-8290.cd-14-0001
Winsor CP (1932) The Gompertz curve as a growth curve. Proc Natl Acad Sci USA 18(1):1–8
Laird AK (1969) Dynamics of growth in tumors and in normal organisms. Natl Cancer Inst Monogr 30:15–28
Law LW (1952) Origin of the resistance of leukaemic cells to folic acid antagonists. Nature 169(4302):628–629
Markov AA (2006) An example of statistical investigation of the text Eugene Onegin concerning the connection of samples in chains. Sci Context 19(4):591
Frobenius G (1912) Über Matrizen Aus Nicht Negativen Elementen. Walter De Gruyter Incorporated
Perron O (1907) Zur Theorie der Matrices. Math Ann 64(2):248–263. doi:10.1007/BF01449896
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES (2011) Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 146(4):633–644. doi:10.1016/j.cell.2011.07.026
Komarova N (2006) Stochastic modeling of drug resistance in cancer. J Theor Biol 239(3):351–366. doi:10.1016/j.jtbi.2005.08.003
Karlin S (1968) A first course in stochastic processes. Academic Press, New York. http://nla.gov.au/nla.cat-vn561499
Sehl M, Zhou H, Sinsheimer JS, Lange KL (2011) Extinction models for cancer stem cell therapy. Math Biosci 234(2):132–146. doi:10.1016/j.mbs.2011.09.005
Kimmel M, Axelrod DE (1991) Unequal cell division, growth regulation and colony size of mammalian cells: a mathematical model and analysis of experimental data. J Theor Biol 153(2):157–180
Jagers P (1975) Branching processes with biological applications. Wiley, London
Harris TE (2002) The theory of branching processes. Dover, New York
Horn M, Glauche I, Müller MC, Hehlmann R, Hochhaus A, Loeffler M, Roeder I (2013) Model-based decision rules reduce the risk of molecular relapse after cessation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Blood 121:378–384. doi:10.1182/blood-2012-07-441956
Loeffler M, Wichmann HE (1980) A comprehensive mathematical model of stem cell proliveration which reproduces most of the published experimental results. Cell Prolif 13(5):543–561. doi:10.1111/j.1365-2184.1980.tb00494.x
Roeder I, Loeffler M (2002) A novel dynamic model of hematopoietic stem cell organization based on the concept of within-tissue plasticity. Exp Hematol 30(8):853–861. doi:10.1016/S0301-472X(02)00832-9
Spencer SL, Berryman MJ, Garcia JA, Abbott D (2004) An ordinary differential equation model for the multistep transformation to cancer. J Theor Biol 231(4):515–524. doi:10.1016/j.jtbi.2004.07.006
Ganguly R, Puri IK (2006) Mathematical model for the cancer stem cell hypothesis. Cell Prolif 39(1):3–14. doi:10.1111/j.1365-2184.2006.00369.x
Gentry SN, Jackson TL (2013) A mathematical model of cancer stem cell driven tumor initiation: implications of niche size and loss of homeostatic regulatory mechanisms. PLoS One 8(8):e71128. doi:10.1371/journal.pone.0071128
Tomlinson IP, Bodmer WF (1995) Failure of programmed cell death and differentiation as causes of tumors: some simple mathematical models. Proc Natl Acad Sci USA 92(24):11130–11134
Ashkenazi R, Gentry SN, Jackson TL (2008) Pathways to tumorigenesis—modeling mutation acquisition in stem cells and their progeny. Neoplasia 10(11):1170–1176. doi:10.1593/neo.08572
Komarova NL, Wodarz D (2003) Evolutionary dynamics of mutator phenotypes in cancer: implications for chemotherapy. Cancer Res 63(20):6635–6642
Gentry SN, Ashkenazi R, Jackson TL (2009) A maturity-structured mathematical model of mutation, acquisition in the absence of homeostatic regulation. Math Model Nat Phenom 4(3):156–182
Tello JI (2013) On a mathematical model of tumor growth based on cancer stem cells. Math Biosci Eng 10(1):263–278. doi:10.3934/mbe.2013.10.263
Kansal AR, Torquato S, Harsh IG, Chiocca EA, Deisboeck TS (2000) Cellular automaton of idealized brain tumor growth dynamics. Bio Syst 55(1–3):119–127
Bankhead A 3rd, Magnuson NS, Heckendorn RB (2007) Cellular automaton simulation examining progenitor hierarchy structure effects on mammary ductal carcinoma in situ. J Theor Biol 246(3):491–498. doi:10.1016/j.jtbi.2007.01.011
Scott JG, Hjelmeland AB, Chinnaiyan P, Anderson AR, Basanta D (2014) Microenvironmental variables must influence intrinsic phenotypic parameters of cancer stem cells to affect tumourigenicity. PLoS Comput Biol 10(1):e1003433. doi:10.1371/journal.pcbi.1003433
Enderling H, Hlatky L, Hahnfeldt P (2013) Cancer stem cells: a minor cancer subpopulation that redefines global cancer features. Front Oncol 3:76. doi:10.3389/fonc.2013.00076
Enderling H (2015) Cancer stem cells: small subpopulation or evolving fraction? Integr Biol 7(1):14–23. doi:10.1039/c4ib00191e
Poleszczuk J, Hahnfeldt P, Enderling H (2015) Evolution and phenotypic selection of cancer stem cells. PLoS Comput Biol 11(3):e1004025. doi:10.1371/journal.pcbi.1004025
Durrett R, Foo J, Leder K (2015) Spatial Moran models, II: cancer initiation in spatially structured tissue. J Math Biol. doi:10.1007/s00285-015-0912-1
Ryser MD, Myers ER, Durrett R (2015) HPV clearance and the neglected role of stochasticity. PLoS Comput Biol 11(3):e1004113. doi:10.1371/journal.pcbi.1004113
Jain HV, Nor JE, Jackson TL (2008) Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral agiogenesis. Bull Math Biol 70(1):89–117. doi:10.1007/s11538-007-9242-9
Jain HV, Nor JE, Jackson TL (2009) Quantification of endothelial cell-targeted anti-Bcl-2 therapy and its suppression of tumor growth and vascularization. Mol Cancer Ther 8(10):2926–2936. doi:10.1158/1535-7163.mct-08-1223
Dong Z, Zeitlin BD, Song W, Sun Q, Karl E, Spencer DM, Jain HV, Jackson T, Nunez G, Nor JE (2007) Level of endothelial cell apoptosis required for a significant decrease in microvessel density. Exp Cell Res 313(16):3645–3657. doi:10.1016/j.yexcr.2007.07.023
Hillen T, Enderling H, Hahnfeldt P (2013) The tumor growth paradox and immune system-mediated selection for cancer stem cells. Bull Math Biol 75(1):161–184. doi:10.1007/s11538-012-9798-x
Enderling H, Anderson AR, Chaplain MA, Beheshti A, Hlatky L, Hahnfeldt P (2009) Paradoxical dependencies of tumor dormancy and progression on basic cell kinetics. Cancer Res 69(22):8814–8821. doi:10.1158/0008-5472.can-09-2115
Imai A, Zeitlin BD, Visioli F, Dong Z, Zhang Z, Krishnamurthy S, Light E, Worden F, Wang S, Nor JE (2012) Metronomic dosing of BH3 mimetic small molecule yields robust antiangiogenic and antitumor effects. Cancer Res 72(3):716–725. doi:10.1158/0008-5472.can-10-2873
Acknowledgments
This work was funded by the University of Michigan Head Neck SPORE P50-CA-97248 (JEN), from the NIH/NCI; R21-DE19279, R01-DE23220 and R01-DE21139 from the NIH/NIDCR (JEN), and a Ruth L. Kirschstein National Research Service Award (NRSA) through the University of Michigan Hematology/Oncology fellowship (T32 2T32CA009357-31A1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pearson, A.T., Jackson, T.L. & Nör, J.E. Modeling head and neck cancer stem cell-mediated tumorigenesis. Cell. Mol. Life Sci. 73, 3279–3289 (2016). https://doi.org/10.1007/s00018-016-2226-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2226-x