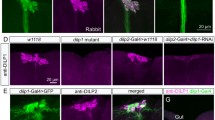
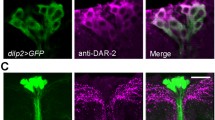
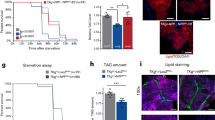
Abstract
Insulin, insulin-like growth factors (IGFs) and insulin-like peptides (ILPs) are important regulators of metabolism, growth, reproduction and lifespan, and mechanisms of insulin/IGF signaling (IIS) have been well conserved over evolution. In insects, between one and 38 ILPs have been identified in each species. Relatively few insect species have been investigated in depth with respect to ILP functions, and therefore we focus mainly on the well-studied fruitfly Drosophila melanogaster. In Drosophila eight ILPs (DILP1-8), but only two receptors (dInR and Lgr3) are known. DILP2, 3 and 5 are produced by a set of neurosecretory cells (IPCs) in the brain and their biosynthesis and release are controlled by a number of mechanisms differing between larvae and adults. Adult IPCs display cell-autonomous sensing of circulating glucose, coupled to evolutionarily conserved mechanisms for DILP release. The glucose-mediated DILP secretion is modulated by neurotransmitters and neuropeptides, as well as by factors released from the intestine and adipocytes. Larval IPCs, however, are indirectly regulated by glucose-sensing endocrine cells producing adipokinetic hormone, or by circulating factors from the intestine and fat body. Furthermore, IIS is situated within a complex physiological regulatory network that also encompasses the lipophilic hormones, 20-hydroxyecdysone and juvenile hormone. After release from IPCs, the ILP action can be modulated by circulating proteins that act either as protective carriers (binding proteins), or competitive inhibitors. Some of these proteins appear to have additional functions that are independent of ILPs. Taken together, the signaling with multiple ILPs is under complex control, ensuring tightly regulated IIS in the organism.




Similar content being viewed by others
References
Banting FG, Best CH (1922) The internal secretion of the pancreas. J Lab Clin Med 7:251–266
Claeys I, Simonet G, Poels J, Van Loy T, Vercammen L, De Loof A, Vanden Broeck J (2002) Insulin-related peptides and their conserved signal transduction pathway. Peptides 23(4):807–816
Antonova Y, Arik AJ, Moore W, Riehle MR, Brown MR (2012) Insulin-like peptides: structure, signaling, and function. In: Gilbert LI (ed) Insect endocrinology. Elsevier/Academic Press, New York, pp 63–92
Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L (2010) Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 6(2):e1000857. doi:10.1371/journal.pgen.1000857
Garofalo RS (2002) Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab 13(4):156–162
Watanabe Y, Miyamoto Y, Matsuda T, Tanaka M (2011) Relaxin-3/INSL7 regulates the stress-response system in the rat hypothalamus. J Mol Neurosci 43(2):169–174. doi:10.1007/s12031-010-9468-0
Lok S, Johnston DS, Conklin D, Lofton-Day CE, Adams RL, Jelmberg AC, Whitmore TE, Schrader S, Griswold MD, Jaspers SR (2000) Identification of INSL6, a new member of the insulin family that is expressed in the testis of the human and rat. Biol Reprod 62(6):1593–1599
Chassin D, Laurent A, Janneau JL, Berger R, Bellet D (1995) Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics 29(2):465–470. doi:10.1006/geno.1995.9980
Froesch ER, Zapf J (1985) Insulin-like growth factors and insulin: comparative aspects. Diabetologia 28(8):485–493
Hudson P, Haley J, John M, Cronk M, Crawford R, Haralambidis J, Tregear G, Shine J, Niall H (1983) Structure of a genomic clone encoding biologically active human relaxin. Nature 301(5901):628–631
Badisco L, Claeys I, Van Hiel M, Clynen E, Huybrechts J, Vandersmissen T, Van Soest S, Vanden Bosch L, Simonet G, Vanden Broeck J (2008) Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J Mol Endocrinol 40(3):137–150. doi:10.1677/JME-07-0161
Yoshida I, Moto K, Sakurai S, Iwami M (1998) A novel member of the bombyxin gene family: structure and expression of bombyxin G1 gene, an insulin-related peptide gene of the silkmoth Bombyx mori. Dev Genes Evol 208(7):407–410
Mizoguchi A, Okamoto N (2013) Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: discovery, structure, secretion, and function. Front Physiol 4:217. doi:10.3389/fphys.2013.00217
Lagueux M, Lwoff L, Meister M, Goltzene F, Hoffmann JA (1990) cDNAs from neurosecretory cells of brains of Locusta migratoria (Insecta, Orthoptera) encoding a novel member of the superfamily of insulins. Eur J Biochem 187(1):249–254
Veenstra JA (2014) The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front Physiol 5:454. doi:10.3389/fphys.2014.00454
Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11(4):213–221
Okamoto N, Nakamori R, Murai T, Yamauchi Y, Masuda A, Nishimura T (2013) A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in Drosophila. Genes Dev 27(1):87–97. doi:10.1101/gad.204479.112
Slaidina M, Delanoue R, Grönke S, Partridge L, Leopold P (2009) A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17(6):874–884. doi:10.1016/j.devcel.2009.10.009
Colombani J, Andersen DS, Leopold P (2012) Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336(6081):582–585. doi:10.1126/science.1216689
Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M (2012) Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336(6081):579–582. doi:10.1126/science.1216735
Vanden Broeck J (2001) Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides 22(2):241–254
Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, Mizoguchi A (2009) A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17(6):885–891. doi:10.1016/j.devcel.2009.10.008
Miguel-Aliaga I, Thor S, Gould AP (2008) Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol 6(3):e58. doi:10.1371/journal.pbio.0060058
Yang CH, Belawat P, Hafen E, Jan LY, Jan YN (2008) Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319(5870):1679–1683. doi:10.1126/science.1151842
Ikeya T, Galic M, Belawat P, Nairz K, Hafen E (2002) Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12(15):1293–1300
Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296(5570):1118–1120. doi:10.1126/science.1070058296/5570/1118
Ward CW, Lawrence MC (2011) Landmarks in insulin research. Front Endocrinol (Lausanne) 2:76. doi:10.3389/fendo.2011.00076
Fernandez AM, Torres-Aleman I (2012) The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 13(4):225–239. doi:10.1038/nrn3209
Van Hiel MB, Vandersmissen HP, Proost P, Vanden Broeck J (2015) Cloning, constitutive activity and expression profiling of two receptors related to relaxin receptors in Drosophila melanogaster. Peptides 68:83–90. doi:10.1016/j.peptides.2014.07.014
Colombani J, Andersn DS, Boulan L, Boone E, Romero N, Virolle V, Tecxada M, Leopold P (2015) Drosophila Lgr3 couples organ growth with maturation and ensures developmental stability. Curr Biol. doi:10.1016/j.cub.2015.09.020
Xu HJ, Xue J, Lu B, Zhang XC, Zhuo JC, He SF, Ma XF, Jiang YQ, Fan HW, Xu JY, Ye YX, Pan PL, Li Q, Bao YY, Nijhout HF, Zhang CX (2015) Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519(7544):464–467. doi:10.1038/nature14286
Boucher P, Ditlecadet D, Dube C, Dufresne F (2010) Unusual duplication of the insulin-like receptor in the crustacean Daphnia pulex. BMC Evol Biol 10:305. doi:10.1186/1471-2148-10-305
Teleman AA (2010) Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425(1):13–26. doi:10.1042/BJ20091181
Goberdhan DC, Wilson C (2003) The functions of insulin signaling: size isn’t everything, even in Drosophila. Differentiation 71(7):375–397
Braeckman BP, Houthoofd K, Vanfleteren JR (2001) Insulin-like signaling, metabolism, stress resistance and aging in Caenorhabditis elegans. Mech Ageing Dev 122(7):673–693
Baumeister R, Schaffitzel E, Hertweck M (2006) Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol 190(2):191–202. doi:10.1677/joe.1.06856
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366(6454):461–464. doi:10.1038/366461a0
Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L (2001) Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292(5514):104–106. doi:10.1126/science.1057991292/5514/104
Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS (2001) A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292(5514):107–110. doi:10.1126/science.1057987292/5514/107
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328(5976):321–326. doi:10.1126/science.1172539
Kenyon C (2005) The plasticity of aging: insights from long-lived mutants. Cell 120(4):449–460. doi:10.1016/j.cell.2005.02.002
McLeod CJ, Wang L, Wong C, Jones DL (2010) Stem cell dynamics in response to nutrient availability. Curr Biol 20(23):2100–2105. doi:10.1016/j.cub.2010.10.038
Sousa-Nunes R, Yee LL, Gould AP (2011) Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471(7339):508–512. doi:10.1038/nature09867
Padmanabha D, Baker KD (2014) Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol Metabol. doi:10.1016/j.tem.2014.03.011
Owusu-Ansah E, Perrimon N (2014) Modeling metabolic homeostasis and nutrient sensing in Drosophila: implications for aging and metabolic diseases. Dis Model Mech 7(3):343–350. doi:10.1242/dmm.012989
Géminard G, Arquier N, Layalle S, Bourouis M, Slaidina M, Delanoue R, Bjordal M, Ohanna M, Ma M, Colombani J, Léopold P (2006) Control of metabolism and growth through insulin-like peptides in Drosophila. Diabetes 55:S5–S8
Giannakou ME, Partridge L (2007) Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci 32(4):180–188. doi:10.1016/j.tibs.2007.02.007
Grewal SS (2009) Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol 41(5):1006–1010. doi:10.1016/j.biocel.2008.10.010
Tatar M, Post S, Yu K (2014) Nutrient control of Drosophila longevity. Trends Endocrinol Metabol 25(10):509–517. doi:10.1016/j.tem.2014.02.006
Sim C, Denlinger DL (2008) Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA 105(18):6777–6781. doi:10.1073/pnas.0802067105
Kubrak OI, Kucerova L, Theopold U, Nassel DR (2014) The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS ONE 9(11):e113051. doi:10.1371/journal.pone.0113051
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Ann Rev Entomol 56:103–121. doi:10.1146/annurev-ento-112408-085436
McElwee JJ, Schuster E, Blanc E, Thornton J, Gems D (2006) Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. Mech Ageing Dev 127(5):458–472. doi:10.1016/j.mad.2006.01.006
Root CM, Ko KI, Jafari A, Wang JW (2011) Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145(1):133–144. doi:10.1016/j.cell.2011.02.008
Crocker A, Shahidullah M, Levitan IB, Sehgal A (2010) Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65(5):670–681. doi:10.1016/j.neuron.2010.01.032
Corl AB, Rodan AR, Heberlein U (2005) Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster. Nat Neurosci 8(1):18–19. doi:10.1038/nn1363
Luo J, Lushchak OV, Goergen P, Williams MJ, Nassel DR (2014) Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior. PLoS ONE 9(6):e99732. doi:10.1371/journal.pone.0099732
Bader R, Sarraf-Zadeh L, Peters M, Moderau N, Stocker H, Kohler K, Pankratz MJ, Hafen E (2013) The IGFBP7 homolog Imp-L2 promotes insulin signaling in distinct neurons of the Drosophila brain. J Cell Sci 126(Pt 12):2571–2576. doi:10.1242/jcs.120261
Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H (2008) Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol 7(3):10. doi:10.1186/jbiol72
Sarraf-Zadeh L, Christen S, Sauer U, Cognigni P, Miguel-Aliaga I, Stocker H, Kohler K, Hafen E (2013) Local requirement of the Drosophila insulin binding protein imp-L2 in coordinating developmental progression with nutritional conditions. Dev Biol 381(1):97–106. doi:10.1016/j.ydbio.2013.06.008
Sloth Andersen A, Hertz Hansen P, Schaffer L, Kristensen C (2000) A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J Biol Chem 275(22):16948–16953. doi:10.1074/jbc.M001578200
Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N (2015) Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell 33(1):36–46. doi:10.1016/j.devcel.2015.02.012
Figueroa-Clarevega A, Bilder D (2015) Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell 33(1):47–55. doi:10.1016/j.devcel.2015.03.001
Cao C, Brown MR (2001) Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res 304(2):317–321
Van de Velde S, Badisco L, Claeys I, Verleyen P, Chen X, Vanden Bosch L, Vanden Broeck J, Smagghe G (2007) Insulin-like peptides in Spodoptera littoralis (Lepidoptera): detection, localization and identification. Gen Comp Endocrinol 153(1–3):72–79. doi:10.1016/j.ygcen.2007.05.001
Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S (2011) Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen Comp Endocrinol 173(2):303–312. doi:10.1016/j.ygcen.2011.06.005
Riehle MA, Fan Y, Cao C, Brown MR (2006) Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides 27(11):2547–2560. doi:10.1016/j.peptides.2006.07.016
Vafopoulou X, Steel CG (2012) Insulin-like and testis ecdysiotropin neuropeptides are regulated by the circadian timing system in the brain during larval-adult development in the insect Rhodnius prolixus (Hemiptera). Gen Comp Endocrinol 179(2):277–288. doi:10.1016/j.ygcen.2012.08.018
Okamoto N, Yamanaka N, Endo Y, Kataoka H, Mizoguchi A (2011) Spatiotemporal patterns of IGF-like peptide expression in the silkmoth Bombyx mori predict its pleiotropic actions. Gen Comp Endocrinol 173(1):171–182. doi:10.1016/j.ygcen.2011.05.009
Okamoto N, Yamanaka N, Satake H, Saegusa H, Kataoka H, Mizoguchi A (2009) An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J 276(5):1221–1232. doi:10.1111/j.1742-4658.2008.06859.x
Veenstra JA, Agricola HJ, Sellami A (2008) Regulatory peptides in fruit fly midgut. Cell Tissue Res 334(3):499–516. doi:10.1007/s00441-008-0708-3
Söderberg JA, Birse RT, Nässel DR (2011) Insulin production and signaling in renal tubules of Drosophila is under control of tachykinin-related peptide and regulates stress resistance. PLoS ONE 6(5):e19866. doi:10.1371/journal.pone.0019866PONE-D-11-03456
Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, Partridge L (2010) DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell 9(3):336–346. doi:10.1111/j.1474-9726.2010.00558.x
Geminard C, Rulifson EJ, Leopold P (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10(3):199–207. doi:10.1016/j.cmet.2009.08.002
Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ (2011) A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4(6):842–849. doi:10.1242/dmm.007948
Nässel DR, Liu Y, Luo J (2015) Insulin/IGF signaling and its regulation in Drosophila. Gen Comp Endocrinol. doi:10.1016/j.ygcen.2014.11.021 (in press)
Kim J, Neufeld TP (2015) Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat Commun 6:6846. doi:10.1038/ncomms7846
Söderberg JA, Carlsson MA, Nässel DR (2012) Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, Drosulfakinin. Front Endocrinol 3:109. doi:10.3389/fendo.2012.00109
Rorsman P, Braun M (2013) Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75:155–179. doi:10.1146/annurev-physiol-030212-183754
Rorsman P, Renstrom E (2003) Insulin granule dynamics in pancreatic beta cells. Diabetologia 46(8):1029–1045. doi:10.1007/s00125-003-1153-1
Prentki M, Matschinsky FM, Madiraju SR (2013) Metabolic signaling in fuel-induced insulin secretion. Cell Metab 18(2):162–185. doi:10.1016/j.cmet.2013.05.018
Nässel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV (2013) Factors that regulate insulin producing cells and their output in Drosophila. Front Physiol 4:252. doi:10.3389/fphys.2013.00252
Okamoto N, Yamanaka N (2015) Nutrition-dependent control of insect development by insulin-like peptides. Curr Opin Insect Sci 11:21–30
Park S, Alfa RW, Topper SM, Kim GE, Kockel L, Kim SK (2014) A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet 10(8):e1004555. doi:10.1371/journal.pgen.1004555
Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C, Scantling D, Mulkey DK, Helfand SL (2009) Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 1(8):699–713
Enell LE, Kapan N, Soderberg JA, Kahsai L, Nässel DR (2010) Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila. PLoS ONE 5(12):e15780. doi:10.1371/journal.pone.0015780
Rajan A, Perrimon N (2012) Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151(1):123–137. doi:10.1016/j.cell.2012.08.019
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13(3):332–339. doi:10.1038/nm1557
Kwak SJ, Hong SH, Bajracharya R, Yang SY, Lee KS, Yu K (2013) Drosophila adiponectin receptor in insulin producing cells regulates glucose and lipid metabolism by controlling insulin secretion. PLoS ONE 8(7):e68641. doi:10.1371/journal.pone.0068641
Bai H, Kang P, Tatar M (2012) Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 11(6):978–985. doi:10.1111/acel.12000
Kapan N, Lushchak OV, Luo J, Nässel DR (2012) Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci 69:4051–4066. doi:10.1007/s00018-012-1097-z
Miyamoto T, Slone J, Song X, Amrein H (2012) A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 151(5):1113–1125. doi:10.1016/j.cell.2012.10.024
Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA, Dow JA, Taghert PH (2005) A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol 208(Pt 7):1239–1246. doi:10.1242/jeb.01529
Birse RT, Söderberg JA, Luo J, Winther ÅM, Nässel DR (2011) Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J Exp Biol 214:4201–4208. doi:10.1242/jeb.062091
Siviter RJ, Coast GM, Winther ÅM, Nachman RJ, Taylor CA, Shirras AD, Coates D, Isaac RE, Nässel DR (2000) Expression and functional characterization of a Drosophila neuropeptide precursor with homology to mammalian preprotachykinin A. J Biol Chem 275(30):23273–23280. doi:10.1074/jbc.M002875200
Hentze JL, Carlsson MA, Kondo S, Nässel DR, Rewitz KF (2015) The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila. Sci Rep 5:11680. doi:10.1038/srep11680
Yoon JG, Stay B (1995) Immunocytochemical localization of Diploptera punctata allatostatin-like peptide in Drosophila melanogaster. J Comp Neurol 363(3):475–488. doi:10.1002/cne.903630310
Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ (2008) Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab 7(4):321–332. doi:10.1016/j.cmet.2008.02.012
Kim SK, Rulifson EJ (2004) Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431(7006):316–320. doi:10.1038/nature02897
Ren GR, Hauser F, Rewitz KF, Kondo S, Engelbrecht AF, Didriksen AK, Schjott SR, Sembach FE, Li S, Sogaard KC, Sondergaard L, Grimmelikhuijzen CJ (2015) CCHamide-2 is an orexigenic brain-gut peptide in Drosophila. PLoS ONE 10(7):e0133017. doi:10.1371/journal.pone.0133017
Sano H, Nakamura A, Texada MJ, Truman JW, Ishimoto H, Kamikouchi A, Nibu Y, Kume K, Ida T, Kojima M (2015) The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster. PLoS Genet 11(5):e1005209. doi:10.1371/journal.pgen.1005209
Veenstra JA, Ida T (2014) More Drosophila enteroendocrine peptides: orcokinin B and the CCHamides 1 and 2. Cell Tissue Res 357(3):607–621. doi:10.1007/s00441-014-1880-2
Alfa RW, Park S, Skelly KR, Poffenberger G, Jain N, Gu X, Kockel L, Wang J, Liu Y, Powers AC, Kim SK (2015) Suppression of insulin production and secretion by a decretin hormone. Cell Metab 21(2):323–333. doi:10.1016/j.cmet.2015.01.006
Cazzamali G, Torp M, Hauser F, Williamson M, Grimmelikhuijzen CJ (2005) The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem Biophys Res Commun 335(1):14–19. doi:10.1016/j.bbrc.2005.07.038
Nässel DR, Winther ÅM (2010) Drosophila neuropeptides in regulation of physiology and behavior. Progr Neurobiol 92(1):42–104. doi:10.1016/j.pneurobio.2010.04.010
Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JA (2002) Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 282(5):R1297–R1307
Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP (2008) A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes Dev 22(14):1877–1893. doi:10.1101/gad.1670508
Luo J, Becnel J, Nichols CD, Nässel DR (2012) Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT(1A) receptor. Cell Mol Life Sci 69(3):471–484. doi:10.1007/s00018-011-0789-0
El-Kholy S, Stephano F, Li Y, Bhandari A, Fink C, Roeder T (2015) Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res 361(3):669–684. doi:10.1007/s00441-015-2137-4
Lushchak OV, Carlsson MA, Nässel DR (2015) Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol Life Sci 72(16):3143–3155. doi:10.1007/s00018-015-1884-4
Bednarova A, Kodrik D, Krishnan N (2013) Unique roles of glucagon and glucagon-like peptides: parallels in understanding the functions of adipokinetic hormones in stress responses in insects. Comp Biochem Physiol A: Mol Integr Physiol 164(1):91–100. doi:10.1016/j.cbpa.2012.10.012
Baumbach J, Xu Y, Hehlert P, Kuhnlein RP (2014) Galphaq, Ggamma1 and Plc21C control Drosophila body fat storage. J Genet Genomics 41(5):283–292. doi:10.1016/j.jgg.2014.03.005
Van der Horst DJ, Van Marrewijk WJ, Diederen JH (2001) Adipokinetic hormones of insect: release, signal transduction, and responses. Int Rev Cytol 211:179–240
Sheldon AL, Zhang J, Fei H, Levitan IB (2011) SLOB, a SLOWPOKE channel binding protein, regulates insulin pathway signaling and metabolism in Drosophila. PLoS ONE 6(8):e23343. doi:10.1371/journal.pone.0023343PONE-D-11-07508
Varghese J, Lim SF, Cohen SM (2010) Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev 24(24):2748–2753. doi:10.1101/gad.1995910
Suh YS, Bhat S, Hong SH, Shin M, Bahk S, Cho KS, Kim SW, Lee KS, Kim YJ, Jones WD, Yu K (2015) Genome-wide microRNA screening reveals that the evolutionary conserved miR-9a regulates body growth by targeting sNPFR1/NPYR. Nat Commun 6:7693. doi:10.1038/ncomms8693
Teleman AA, Maitra S, Cohen SM (2006) Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev 20(4):417–422. doi:10.1101/gad.374406
Lee GJ, Jun JW, Hyun S (2015) MicroRNA miR-8 regulates multiple growth factor hormones produced from Drosophila fat cells. Insect Mol Biol 24(3):311–318. doi:10.1111/imb.12156
Luhur A, Chawla G, Sokol NS (2013) MicroRNAs as components of systemic signaling pathways in Drosophila melanogaster. Curr Top Dev Biol 105:97–123. doi:10.1016/B978-0-12-396968-2.00004-X
Badisco L, Van Wielendaele P, Vanden Broeck J (2013) Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front Physiol 4:202
Hansen IA, Attardo GM, Rodriguez SD, Drake LL (2014) Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol 5:103. doi:10.3389/fphys.2014.00103
Fallon AM, Hagedorn HH, Wyatt GR, Laufer H (1974) Activation of vitellogenin synthesis in the mosquito Aedes aegypti by ecdysone. J Insect Physiol 20(9):1815–1823
Martin D, Wang SF, Raikhel AS (2001) The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol Cell Endocrinol 173(1–2):75–86
Riehle MA, Brown MR (1999) Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem Mol Biol 29(10):855–860
Riehle MA, Brown MR (2002) Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tissue Res 308(3):409–420. doi:10.1007/s00441-002-0561-8
Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR (2008) An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA 105(15):5716–5721. doi:10.1073/pnas.0800478105
Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR, Brown MR (2010) Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol 328(1–2):47–55. doi:10.1016/j.mce.2010.07.003
Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO (1998) Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem 273(7):3967–3971
Tu MP, Yin CM, Tatar M (2005) Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol 142(3):347–356. doi:10.1016/j.ygcen.2005.02.009
Mutti NS, Dolezal AG, Wolschin F, Mutti JS, Gill KS, Amdam GV (2011) IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J Exp Biol 214(Pt 23):3977–3984. doi:10.1242/jeb.061499
Suren-Castillo S, Abrisqueta M, Maestro JL (2012) FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem Mol Biol 42(7):491–498. doi:10.1016/j.ibmb.2012.03.006
Perez-Hedo M, Rivera-Perez C, Noriega FG (2013) The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochem Mol Biol 43(6):495–500. doi:10.1016/j.ibmb.2013.03.008
Sim C, Denlinger DL (2013) Insulin signaling and the regulation of insect diapause. Front Physiol 4:189. doi:10.3389/fphys.2013.00189
Abrisqueta M, Suren-Castillo S, Maestro JL (2014) Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem Mol Biol 49:14–23. doi:10.1016/j.ibmb.2014.03.005
Sheng Z, Xu J, Bai H, Zhu F, Palli SR (2011) Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J Biol Chem 286(49):41924–41936. doi:10.1074/jbc.M111.269845
Parthasarathy R, Palli SR (2011) Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 41(5):294–305. doi:10.1016/j.ibmb.2011.01.006
Xu J, Sheng Z, Palli SR (2013) Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS genetics 9(6):e1003535. doi:10.1371/journal.pgen.1003535
Badisco L, Marchal E, Van Wielendaele P, Verlinden H, Vleugels R, Vanden Broeck J (2011) RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust Schistocerca gregaria. Peptides 32(3):573–580. doi:10.1016/j.peptides.2010.11.008
Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC (2012) A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337(6096):860–864. doi:10.1126/science.1224286
Riddiford LM (2012) How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol 179(3):477–484. doi:10.1016/j.ygcen.2012.06.001
Koyama T, Mendes CC, Mirth CK (2013) Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front Physiol 4:263. doi:10.3389/fphys.2013.00263
Lozano J, Belles X (2014) Role of Methoprene-tolerant (Met) in adult morphogenesis and in adult ecdysis of Blattella germanica. PLoS ONE 9(7):e103614. doi:10.1371/journal.pone.0103614
Mirth CK, Tang HY, Makohon-Moore SC, Salhadar S, Gokhale RH, Warner RD, Koyama T, Riddiford LM, Shingleton AW (2014) Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc Natl Acad Sci USA 111(19):7018–7023. doi:10.1073/pnas.1313058111
Smith WA, Lamattina A, Collins M (2014) Insulin signaling pathways in lepidopteran ecdysone secretion. Front Physiol 5:19. doi:10.3389/fphys.2014.00019
Tsuda M, Kobayashi T, Matsuo T, Aigaki T (2010) Insulin-degrading enzyme antagonizes insulin-dependent tissue growth and Abeta-induced neurotoxicity in Drosophila. FEBS Lett 584(13):2916–2920. doi:10.1016/j.febslet.2010.05.010
Hyun J, Hashimoto C (2011) Physiological effects of manipulating the level of insulin-degrading enzyme in insulin-producing cells of Drosophila. Fly (Austin) 5(1):53–57
Jones JI, Clemmons DR (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16(1):3–34
Russo VC, Gluckman PD, Feldman EL, Werther GA (2005) The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev 26(7):916–943. doi:10.1210/er.2004-0024
Baxter RC (2014) IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer 14(5):329–341. doi:10.1038/nrc3720
Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE (2005) Molecular interactions of the IGF system. Cytokine Growth Factor Rev 16(4–5):421–439. doi:10.1016/j.cytogfr.2005.04.004
Hwa V, Oh Y, Rosenfeld RG (1999) The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 20(6):761–787. doi:10.1210/edrv.20.6.0382
Forbes BE, McCarthy P, Norton RS (2012) Insulin-like growth factor binding proteins: a structural perspective. Front Endocrinol (Lausanne) 3:38. doi:10.3389/fendo.2012.00038
Beattie J, Kreiner M, Allan GJ, Flint DJ, Domingues D, van der Walle CF (2009) IGFBP-3 and IGFBP-5 associate with the cell binding domain (CBD) of fibronectin. Biochem Biophys Res Commun 381(4):572–576. doi:10.1016/j.bbrc.2009.02.088
Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, Sorensen PH, Seth A (2012) IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal 5(255):ra92. doi:10.1126/scisignal.2003184
Hwang JR, Huh JH, Lee Y, Lee SI, Rho SB, Lee JH (2011) Insulin-like growth factor-binding protein-5 (IGFBP-5) inhibits TNF-α-induced NF-κB activity by binding to TNFR1. Biochem Biophys Res Commun 405(4):545–551. doi:10.1016/j.bbrc.2011.01.064
Kawai M, Breggia AC, DeMambro VE, Shen X, Canalis E, Bouxsein ML, Beamer WG, Clemmons DR, Rosen CJ (2011) The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem 286(16):14670–14680. doi:10.1074/jbc.M110.193334
Miljus G, Malenkovic V, Dukanovic B, Kolundzic N, Nedic O (2015) IGFBP-3/transferrin/transferrin receptor 1 complexes as principal mediators of IGFBP-3 delivery to colon cells in non-cancer and cancer tissues. Exp Mol Pathol 98(3):431–438. doi:10.1016/j.yexmp.2015.03.035
Zhong Y, Lu L, Zhou J, Li Y, Liu Y, Clemmons DR, Duan C (2011) IGF binding protein 3 exerts its ligand-independent action by antagonizing BMP in zebrafish embryos. J Cell Sci 124(Pt 11):1925–1935. doi:10.1242/jcs.082644
Azar WJ, Zivkovic S, Werther GA, Russo VC (2014) IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene 33(5):578–588. doi:10.1038/onc.2012.630
Dai W, Kamei H, Zhao Y, Ding J, Du Z, Duan C (2010) Duplicated zebrafish insulin-like growth factor binding protein-5 genes with split functional domains: evidence for evolutionarily conserved IGF binding, nuclear localization, and transactivation activity. Faseb J 24(6):2020–2029. doi:10.1096/fj.09-149435
Zhou J, Xiang J, Zhang S, Duan C (2013) Structural and functional analysis of the amphioxus IGFBP gene uncovers ancient origin of IGF-independent functions. Endocrinology 154(10):3753–3763. doi:10.1210/en.2013-1201
Daza DO, Sundstrom G, Bergqvist CA, Duan C, Larhammar D (2011) Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology 152(6):2278–2289. doi:10.1210/en.2011-0047
Osterbur DL, Fristrom DK, Natzle JE, Tojo SJ, Fristrom JW (1988) Genes expressed during imaginal discs morphogenesis: IMP-L2, a gene expressed during imaginal disc and imaginal histoblast morphogenesis. Dev Biol 129(2):439–448
Zapf J, Schoenle E, Froesch ER (1985) In vivo effects of the insulin-like growth factors (IGFs) in the hypophysectomized rat: comparison with human growth hormone and the possible role of the specific IGF carrier proteins. Ciba Found Symp 116:169–187
Garbe JC, Yang E, Fristrom JW (1993) IMP-L2: an essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development 119(4):1237–1250
Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M (2008) Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci USA 105(17):6368–6373. doi:10.1073/pnas.0709128105
Alic N, Hoddinott MP, Vinti G, Partridge L (2011) Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 10(1):137–147. doi:10.1111/j.1474-9726.2010.00653.x
Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P (2008) Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab 7(4):333–338. doi:10.1016/j.cmet.2008.02.003
Weiss IM, Gohring W, Fritz M, Mann K (2001) Perlustrin, a Haliotis laevigata (abalone) nacre protein, is homologous to the insulin-like growth factor binding protein N-terminal module of vertebrates. Biochem Biophys Res Commun 285(2):244–249. doi:10.1006/bbrc.2001.5170
Rosen O, Weil S, Manor R, Roth Z, Khalaila I, Sagi A (2013) A crayfish insulin-like-binding protein: another piece in the androgenic gland insulin-like hormone puzzle is revealed. J Biol Chem 288(31):22289–22298. doi:10.1074/jbc.M113.484279
Claeys I, Simonet G, Van Loy T, De Loof A, Vanden Broeck J (2003) cDNA cloning and transcript distribution of two novel members of the neuroparsin family in the desert locust, Schistocerca gregaria. Insect Mol Biol 12(5):473–481
Badisco L, Claeys I, Van Loy T, Van Hiel M, Franssens V, Simonet G, Vanden Broeck J (2007) Neuroparsins, a family of conserved arthropod neuropeptides. Gen Comp Endocrinol 153(1–3):64–71. doi:10.1016/j.ygcen.2007.03.008
Huang X, Ye H, Feng B, Huang H (2015) Insights into insulin-like peptide system in invertebrates from studies on IGF binding domain-containing proteins in the female Mud Crab, Scylla paramamosain. Mol Cell Endocrinol. doi:10.1016/j.mce.2015.08.019
Girardie J, Girardie A, Huet JC, Pernollet JC (1989) Amino acid sequence of locust neuroparsins. FEBS Lett 245(1–2):4–8
Girardie J, Bourême D, Couillaud F, Tamarelle M, Girardie A (1987) Anti-juvenile effect of neuroparsin-A, A neuroprotein isolated from locust corpora cardiaca. Insect Biochem 17:977–983
Janssen T, Claeys I, Simonet G, De Loof A, Girardie J, Vanden Broeck J (2001) cDNA cloning and transcript distribution of two different neuroparsin precursors in the desert locust, Schistocerca gregaria. Insect Mol Biol 10(2):183–189
Veenstra JA (2014) The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front Physiol 5:454. doi:10.3389/fphys.2014.00454
Veenstra JA (2010) What the loss of the hormone neuroparsin in the melanogaster subgroup of Drosophila can tell us about its function. Insect Biochem Mol Biol 40(4):354–361. doi:10.1016/j.ibmb.2010.03.001
Dhara A, Eum JH, Robertson A, Gulia-Nuss M, Vogel KJ, Clark KD, Graf R, Brown MR, Strand MR (2013) Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol 43(12):1100–1108. doi:10.1016/j.ibmb.2013.09.004
Vogel KJ, Brown MR, Strand MR (2015) Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc Natl Acad Sci USA 112(16):5057–5062. doi:10.1073/pnas.1501814112
Dissous C (2015) Venus kinase receptors at the crossroads of insulin signaling: their role in reproduction for helminths and insects. Front Endocrinol (Lausanne) 6:118. doi:10.3389/fendo.2015.00118
Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, Mizoguchi A, Fujiwara Y, Takahashi SY, Ishizaki H (1986) Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori. Proc Natl Acad Sci USA 83(16):5840–5843
Hetru C, Li KW, Bulet P, Lagueux M, Hoffmann JA (1991) Isolation and structural characterization of an insulin-related molecule, a predominant neuropeptide from Locusta migratoria. Eur J Biochem 201(2):495–499
Cobelli C, Pacini G (1988) Insulin secretion and hepatic extraction in humans by minimal modeling of C-peptide and insulin kinetics. Diabetes 37(2):223–231
Steiner DF (2004) The proinsulin C-peptide–a multirole model. Exp Diabesity Res 5(1):7–14. doi:10.1080/15438600490424389
Bermudez I, Beadle DJ, Trifilieff E, Luu B, Hietter H (1991) Electrophysiological activity of the C-peptide of the Locusta insulin-related peptide. Effect on the membrane conductance of Locusta neurones in vitro. FEBS Lett 293(1–2):137–141
Clynen E, Huybrechts J, Baggerman G, Van Doorn J, Van Der Horst D, De Loof A, Schoofs L (2003) Identification of a glycogenolysis-inhibiting peptide from the corpora cardiaca of locusts. Endocrinology 144(8):3441–3448. doi:10.1210/en.2002-0107
Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J (1995) The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J 14(14):3373–3384
Sajid W, Kulahin N, Schluckebier G, Ribel U, Henderson HR, Tatar M, Hansen BF, Svendsen AM, Kiselyov VV, Norgaard P, Wahlund PO, Brandt J, Kohanski RA, Andersen AS, De Meyts P (2011) Structural and biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J Biol Chem 286(1):661–673. doi:10.1074/jbc.M110.156018
Veenstra JA (2015) The power of next-generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii. Gen Comp Endocrinol. doi:10.1016/j.ygcen.2015.06.013
Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L (2008) Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 3(11):e3721. doi:10.1371/journal.pone.0003721
Toivonen JM, Partridge L (2009) Endocrine regulation of aging and reproduction in Drosophila. Mol Cell Endocrinol 299(1):39–50. doi:10.1016/j.mce.2008.07.005
Williams MJ, Eriksson A, Shaik M, Voisin S, Yamskova O, Paulsson J, Thombare K, Fredriksson R, Schioth HB (2015) The obesity-linked gene Nudt3 Drosophila homolog Aps is associated with insulin signalling. Mol Endocrinol. doi:10.1210/ME.2015-1077
Karpac J, Hull-Thompson J, Falleur M, Jasper H (2009) JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell 8(3):288–295. doi:10.1111/j.1474-9726.2009.00476.x
Docherty JE, Manno JE, McDermott JE, DiAngelo JR (2015) Mio acts in the Drosophila brain to control nutrient storage and feeding. Gene 568(2):190–195. doi:10.1016/j.gene.2015.05.055
Sekine O, Love DC, Rubenstein DS, Hanover JA (2010) Blocking O-linked GlcNAc cycling in Drosophila insulin-producing cells perturbs glucose-insulin homeostasis. J Biol Chem 285(49):38684–38691. doi:10.1074/jbc.M110.155192
Clements J, Hens K, Francis C, Schellens A, Callaerts P (2008) Conserved role for the Drosophila Pax6 homolog eyeless in differentiation and function of insulin-producing neurons. Proc Natl Acad Sci USA 105(42):16183–16188. doi:10.1073/pnas.0708330105
Cao J, Ni J, Ma W, Shiu V, Milla LA, Park S, Spletter ML, Tang S, Zhang J, Wei X, Kim SK, Scott MP (2014) Insight into insulin secretion from transcriptome and genetic analysis of insulin-producing cells of Drosophila. Genetics 197(1):175–192. doi:10.1534/genetics.113.160663
Davis SM, Thomas AL, Nomie KJ, Huang L, Dierick HA (2014) Tailless and Atrophin control Drosophila aggression by regulating neuropeptide signalling in the pars intercerebralis. Nat Commun 5:3177. doi:10.1038/ncomms4177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nässel, D.R., Broeck, J.V. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 73, 271–290 (2016). https://doi.org/10.1007/s00018-015-2063-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2063-3