Abstract
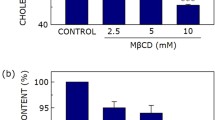
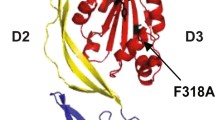
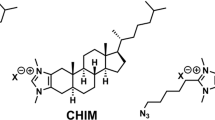
Although cholesterol is essential for membrane fluidity and deformability, the level of its lateral heterogeneity at the plasma membrane of living cells is poorly understood due to lack of appropriate probe. We here report on the usefulness of the D4 fragment of Clostridium perfringens toxin fused to mCherry (theta*), as specific, non-toxic, sensitive and quantitative cholesterol-labeling tool, using erythrocyte flat membrane. By confocal microscopy, theta* labels cholesterol-enriched submicrometric domains in coverslip-spread but also gel-suspended (non-stretched) fresh erythrocytes, suggesting in vivo relevance. Cholesterol domains on spread erythrocytes are stable in time and space, restricted by membrane:spectrin anchorage via 4.1R complexes, and depend on temperature and sphingomyelin, indicating combined regulation by extrinsic membrane:cytoskeleton interaction and by intrinsic lipid packing. Cholesterol domains partially co-localize with BODIPY-sphingomyelin-enriched domains. In conclusion, we show that theta* is a useful vital probe to study cholesterol organization and demonstrate that cholesterol forms submicrometric domains in living cells.









Similar content being viewed by others
Abbreviations
- CalA:
-
Calyculin A
- DF-BSA:
-
Defatted-bovine serum albumin
- FRAP:
-
Fluorescence recovery after photobleaching
- LatB:
-
Latrunculin B
- mβCD:
-
Methyl-β-cyclodextrin
- MLVs:
-
Multilamellar vesicles
- PC:
-
Phosphatidylcholine
- PKC:
-
Protein kinase C
- PMA:
-
Phorbol 12-myristate 13-acetate
- RBC:
-
Red blood cell
- SEM:
-
Scanning electron microscopy
- SM:
-
Sphingomyelin
- SMase:
-
Sphingomyelinase
- theta*:
-
His-mCherry-theta-D4
References
Lange Y, Swaisgood MH, Ramos BV, Steck TL (1989) Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem 264:3786–3793
Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327:46–50
Pike LJ (2006) Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res 47:1597–1598
Parton RG, Way M, Zorzi N, Stang E (1997) Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 136:137–154
Schroeder F, Nemecz G, Wood WG, Joiner C, Morrot G, Ayraut-Jarrier M, Devaux PF (1991) Transmembrane distribution of sterol in the human erythrocyte. Biochim Biophys Acta 1066:183–192
Lange Y, Slayton JM (1982) Interaction of cholesterol and lysophosphatidylcholine in determining red cell shape. J Lipid Res 23:1121–1127
Sun M, Northup N, Marga F, Huber T, Byfield FJ, Levitan I, Forgacs G (2007) The effect of cellular cholesterol on membrane-cytoskeleton adhesion. J Cell Sci 120:2223–2231
Laurenzana A, Fibbi G, Chilla A, Margheri G, Del Rosso T, Rovida E, Del Rosso M, Margheri F (2015) Lipid rafts: integrated platforms for vascular organization offering therapeutic opportunities. Cell Mol Life Sci 72:1537–1557
Baumann P, Thiele W, Cremers N, Muppala S, Krachulec J, Diefenbacher M, Kassel O, Mudduluru G, Allgayer H, Frame M, Sleeman JP (2012) CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion. Cell Mol Life Sci 69:435–448
Chichili GR, Rodgers W (2009) Cytoskeleton-membrane interactions in membrane raft structure. Cell Mol Life Sci 66:2319–2328
Veiga MP, Arrondo JL, Goni FM, Alonso A, Marsh D (2001) Interaction of cholesterol with sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry 40:2614–2622
Fidorra M, Heimburg T, Bagatolli LA (2009) Direct visualization of the lateral structure of porcine brain cerebrosides/POPC mixtures in presence and absence of cholesterol. Biophys J 97:142–154
Bali R, Savino L, Ramirez DA, Tsvetkova NM, Bagatolli L, Tablin F, Crowe JH, Leidy C (2009) Macroscopic domain formation during cooling in the platelet plasma membrane: an issue of low cholesterol content. Biochim Biophys Acta 1788:1229–1237
Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W (2007) Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J 26:1–8
Malinsky J, Opekarova M, Grossmann G, Tanner W (2013) Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annu Rev Plant Biol 64:501–529
Hamilton-Miller JM (1973) Chemistry and biology of the polyene macrolide antibiotics. Bacteriol Rev 37:166–196
Gimpl G, Gehrig-Burger K (2011) Probes for studying cholesterol binding and cell biology. Steroids 76:216–231
Waheed AA, Shimada Y, Heijnen HF, Nakamura M, Inomata M, Hayashi M, Iwashita S, Slot JW, Ohno-Iwashita Y (2001) Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc Natl Acad Sci USA 98:4926–4931
Shimada Y, Maruya M, Iwashita S, Ohno-Iwashita Y (2002) The C-terminal domain of perfringolysin O is an essential cholesterol-binding unit targeting to cholesterol-rich microdomains. Eur J Biochem 269:6195–6203
Bavdek A, Gekara NO, Priselac D, Gutierrez Aguirre I, Darji A, Chakraborty T, Macek P, Lakey JH, Weiss S, Anderluh G (2007) Sterol and pH interdependence in the binding, oligomerization, and pore formation of Listeriolysin O. Biochemistry 46:4425–4437
Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK (2010) Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci USA 107:4341–4346
Nelson LD, Johnson AE, London E (2008) How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem 283:4632–4642
Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW (1997) Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685–692
Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A (2014) Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. Elife 3:e02882
Das A, Goldstein JL, Anderson DD, Brown MS, Radhakrishnan A (2013) Use of mutant 125I-perfringolysin O to probe transport and organization of cholesterol in membranes of animal cells. Proc Natl Acad Sci USA 110:10580–10585
Ohno-Iwashita Y, Shimada Y, Waheed AA, Hayashi M, Inomata M, Nakamura M, Maruya M, Iwashita S (2004) Perfringolysin O, a cholesterol-binding cytolysin, as a probe for lipid rafts. Anaerobe 10:125–134
Mizuno H, Abe M, Dedecker P, Makino A, Rocha S, Ohno-Iwashita Y, Hofkens J, Kobayashi T, Miyawaki A (2011) Fluorescent probes for superresolution imaging of lipid domains on the plasma membrane. Chem Sci 2:1548–1553
Frisz JF, Klitzing HA, Lou K, Hutcheon ID, Weber PK, Zimmerberg J, Kraft ML (2013) Sphingolipid domains in the plasma membranes of fibroblasts are not enriched with cholesterol. J Biol Chem 288:16855–16861
Frisz JF, Lou K, Klitzing HA, Hanafin WP, Lizunov V, Wilson RL, Carpenter KJ, Kim R, Hutcheon ID, Zimmerberg J, Weber PK, Kraft ML (2013) Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc Natl Acad Sci USA 110:E613–E622
Ziomek CA, Schulman S, Edidin M (1980) Redistribution of membrane proteins in isolated mouse intestinal epithelial cells. J Cell Biol 86:849–857
Grimmer S, van Deurs B, Sandvig K (2002) Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci 115:2953–2962
Carquin M, Pollet H, Veiga-da-Cunha M, Cominelli A, Van Der Smissen P, N’Kuli F, Emonard H, Henriet P, Mizuno H, Courtoy PJ, Tyteca D (2014) Endogenous sphingomyelin segregates into submicrometric domains in the living erythrocyte membrane. J Lipid Res 55:1331–1342
D’Auria L, Fenaux M, Aleksandrowicz P, Van Der Smissen P, Chantrain C, Vermylen C, Vikkula M, Courtoy PJ, Tyteca D (2013) Micrometric segregation of fluorescent membrane lipids: relevance for endogenous lipids and biogenesis in erythrocytes. J Lipid Res 54:1066–1076
D’Auria L, Van Der Smissen P, Bruyneel F, Courtoy PJ, Tyteca D (2011) Segregation of fluorescent membrane lipids into distinct micrometric domains: evidence for phase compartmentation of natural lipids? PLoS One 6:e17021
Tyteca D, D’Auria L, Van Der Smissen P, Medts T, Carpentier S, Monbaliu JC, de Diesbach P, Courtoy PJ (2010) Three unrelated sphingomyelin analogs spontaneously cluster into plasma membrane micrometric domains. Biochim Biophys Acta 1798:909–927
Zachowski A (1993) Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J 294(Pt 1):1–14
Goodman SR, Kurdia A, Ammann L, Kakhniashvili D, Daescu O (2007) The human red blood cell proteome and interactome. Exp Biol Med (Maywood) 232:1391–1408
Salomao M, Zhang X, Yang Y, Lee S, Hartwig JH, Chasis JA, Mohandas N, An X (2008) Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci USA 105:8026–8031
Cooper RA (1978) Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J Supramol Struct 8:413–430
Cornwell DG, Heikkila RE, Bar RS, Biagi GL (1967) Red blood cell lipids and the plasma membrane. JAOCS 45:297–304
Ohno-Iwashita Y, Iwamoto M, Mitsui K, Ando S, Nagai Y (1988) Protease-nicked theta-toxin of Clostridium perfringens, a new membrane probe with no cytolytic effect, reveals two classes of cholesterol as toxin-binding sites on sheep erythrocytes. Eur J Biochem 176:95–101
Betz T, Lenz M, Joanny JF, Sykes C (2009) ATP-dependent mechanics of red blood cells. Proc Natl Acad Sci USA 106:15320–15325
Wustner D (2007) Plasma membrane sterol distribution resembles the surface topography of living cells. Mol Biol Cell 18:211–228
Maekawa M, Fairn GD (2015) Complementary probes reveal that phosphatidylserine is required for the proper transbilayer distribution of cholesterol. J Cell Sci 128:1422–1433
Iwamoto M, Ohno-Iwashita Y, Ando S (1990) Effect of isolated C-terminal fragment of theta-toxin (perfringolysin O) on toxin assembly and membrane lysis. Eur J Biochem 194:25–31
Sokolov A, Radhakrishnan A (2010) Accessibility of cholesterol in endoplasmic reticulum membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold. J Biol Chem 285:29480–29490
McKanna JA, Haigler HT, Cohen S (1979) Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci USA 76:5689–5693
De Roe C, Courtoy PJ, Baudhuin P (1987) A model of protein-colloidal gold interactions. J Histochem Cytochem 35:1191–1198
Cai M, Zhao W, Shang X, Jiang J, Ji H, Tang Z, Wang H (2012) Direct evidence of lipid rafts by in situ atomic force microscopy. Small 8:1243–1250
Bosmann HB, Hagopian A, Eylar EH (1968) Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys 128:51–69
Perkins RG, Scott RE (1978) Plasma membrane phospholipid, cholesterol and fatty acyl composition of differentiated and undifferentiated L6 myoblasts. Lipids 13:334–337
Bezrukov L, Blank PS, Polozov IV, Zimmerberg J (2009) An adhesion-based method for plasma membrane isolation: evaluating cholesterol extraction from cells and their membranes. Anal Biochem 394:171–176
Oglecka K, Rangamani P, Liedberg B, Kraut RS, Parikh AN (2014) Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials. Elife 3:e03695
Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara TK (2011) Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci 36:604–615
Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A (2002) Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol 157:1071–1081
Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KG (2012) Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu Rev Cell Dev Biol 28:215–250
Ehrig J, Petrov EP, Schwille P (2011) Near-critical fluctuations and cytoskeleton-assisted phase separation lead to subdiffusion in cell membranes. Biophys J 100:80–89
Honigmann A, Sadeghi S, Keller J, Hell SW, Eggeling C, Vink R (2014) A lipid bound actin meshwork organizes liquid phase separation in model membranes. Elife 3:e01671
Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144:402–413
Guo Q, Park S, Ma H (2012) Microfluidic micropipette aspiration for measuring the deformability of single cells. Lab Chip 12:2687–2695
Bassereau P, Sorre B, Levy A (2014) Bending lipid membranes: experiments after W. Helfrich’s model. Adv Colloid Interface Sci 208:47–57
Willekens FL, Werre JM, Groenen-Dopp YA, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJ (2008) Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol 141:549–556
Salzer U, Zhu R, Luten M, Isobe H, Pastushenko V, Perkmann T, Hinterdorfer P, Bosman GJ (2008) Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion 48:451–462
Bosman GJ, Lasonder E, Groenen-Dopp YA, Willekens FL, Werre JM (2012) The proteome of erythrocyte-derived microparticles from plasma: new clues for erythrocyte aging and vesiculation. J Proteomics 76:203–210
van Galen J, Campelo F, Martinez-Alonso E, Scarpa M, Martinez-Menarguez JA, Malhotra V (2014) Sphingomyelin homeostasis is required to form functional enzymatic domains at the trans-Golgi network. J Cell Biol 206:609–618
van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, Jahn R (2011) Membrane protein sequestering by ionic protein-lipid interactions. Nature 479:552–555
Acknowledgments
We thank Drs. A. Miyawaki, M. Abe and T. Kobayashi at Riken Brain Science Institute (Saitama, Japan) as well as H. Mizuno (KU Leuven, Belgium) for generously supplying the Dronpa-theta-D4 plasmid, Dr. A. De Matteis (Naples, Italy) for mCherry-Rab5 plasmid and P. Gailly for C2C12 myoblasts; P. Henriet, H. Emonard and J. Lorent for help in producing toxin* and liposomes and T. Lac and N. Chevalier for technical support (UCL, Belgium). This work was supported by grants from UCL (FSR), the F.R.S.-FNRS, Salus Sanguinis foundation, ARC, IUAP and the Région Wallonne. DT and MVdC are Research associates at Fonds de la Recherche Scientifique (F.R.S.-FNRS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carquin, M., Conrard, L., Pollet, H. et al. Cholesterol segregates into submicrometric domains at the living erythrocyte membrane: evidence and regulation. Cell. Mol. Life Sci. 72, 4633–4651 (2015). https://doi.org/10.1007/s00018-015-1951-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1951-x