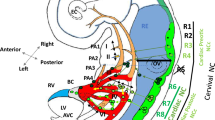
Abstract
Congenital heart defects represent the most common human birth defects and are often life-threatening. Frequently, they are caused by abnormalities of the outflow tract whose formation results from coordinated development of cells from mesodermal and neural crest origin and depends on the activity of many different transcription factors. However, place, time, and mode of action have only been analyzed for a few of them. Here we assess the contribution of the closely related high-mobility-group transcription factors Sox4 and Sox11 to outflow tract development and determine their function. Using cell-type-specific deletion in the mouse, we show that Sox11 is required for proper development in both mesodermal cells and neural crest cells. Deletion in either mesoderm or neural crest, or both, leads to outflow tract defects ranging from double outlet right ventricle to common arterial trunk. Sox4 supports Sox11 in its function, but has additional roles with relevance for outflow tract formation in other cell types. The two Sox proteins are dispensable during early phases of cardiac neural crest development including neural tube emigration, proliferation, and migration through the pharyngeal arches. They become essential after arrival of the neural crest cells in the outflow tract for their proper differentiation and interaction with each other as well as with the environment through regulation of cytoskeletal, cell adhesion, and extracellular matrix molecules. Our results demonstrate that Sox4 and Sox11 have multiple functions in several cell types during outflow tract formation and may thus help to understand the basis of congenital heart defects in humans.








Similar content being viewed by others
References
Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39:1890–1900
Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK (2003) Septation and separation within the outflow tract of the developing heart. J Anat 202:327–342
Snider P, Olaopa M, Firulli AB, Conway SJ (2007) Cardiovascular development and the colonizing cardiac neural crest lineage. Sci World J 7:1090–1113
Srivastava D (2006) Making or breaking the heart: from lineage determination to morphogenesis. Cell 126:1037–1048
Kodo K, Yamagishi H (2011) A decade of advances in the molecular embryology and genetics underlying congenital heart defects. Circ J 75:2296–2304
Schilham MW, Oosterwegel MA, Moerer P, Ya J, Deboer PAJ, Vandewetering M, Verbeek S, Lamers WH, Kruisbeek AM, Cumano A, Clevers H (1996) Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature 380:711–714
Sock E, Rettig SD, Enderich J, Bösl MR, Tamm ER, Wegner M (2004) Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol 24:6635–6644
Thein DC, Thalhammer JM, Hartwig AC, Crenshaw EB 3rd, Lefebvre V, Wegner M, Sock E (2010) The closely related transcription factors Sox4 and Sox11 function as survival factors during spinal cord development. J Neurochem 115:131–141
Wegner M (1999) From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27:1409–1420
Bhattaram P, Penzo-Méndez A, Sock E, Colmenares C, Kaneko KJ, DePamphilis ML, Wegner M, Lefebvre V (2010) Organogenesis relies on Sox4, Sox11, and Sox12 for survival of neural and mesenchymal progenitor cells. Nat Commun 1:9
Penzo-Mendez A, Dy P, Pallavi B, Lefebvre V (2007) Generation of mice harboring a Sox4 conditional null allele. Genesis 45:776–780
Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4
Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 8:1323–1326
Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP (2002) Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nk2–5. Int J Dev Biol 46:431–439
Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM (2003) Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development 130:6361–6374
Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M (2001) Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230:230–242
Hayashi S, Lewis P, Pevny L, McMahon AP (2002) Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns 2:93–97
Hoser M, Baader SL, Bosl MR, Ihmer A, Wegner M, Sock E (2007) Prolonged glial expression of Sox4 in the CNS leads to architectural cerebellar defects and ataxia. J Neurosci 27:5495–5505
Kuhlbrodt K, Herbarth B, Sock E, Enderich J, Hermans-Borgmeyer I, Wegner M (1998) Cooperative function of POU proteins and Sox proteins in glial cells. J Biol Chem 273:16050–16057
Ehrnsperger A, Rehli M, Thu-Hang P, Kreutz M (2005) Epigenetic regulation of the dendritic cell-marker gene ADAM19. Biochem Biophys Res Commun 332:456–464
Peirano RI, Goerich DE, Riethmacher D, Wegner M (2000) Protein zero expression is regulated by the glial transcription factor Sox10. Mol Cell Biol 20:3198–3209
Kellerer S, Schreiner S, Stolt CC, Bösl MR, Wegner M (2006) Replacement of the Sox10 transcription factor by Sox8 reveals incomplete functional equivalence. Development 133:2875–2886
Schlierf B, Werner T, Glaser G, Wegner M (2006) Expression of Connexin47 in oligodendrocytes is regulated by the Sox10 transcription factor. J Mol Biol 361:11–21
Olaopa M, Zhou HM, Snider P, Wang J, Schwartz RJ, Moon AM, Conway SJ (2011) Pax3 is essential for normal cardiac neural crest morphogenesis but is not required during migration nor outflow tract septation. Dev Biol 356:308–322
Hoser M, Potzner MR, Koch JMC, Bösl MR, Wegner M, Sock E (2008) Sox12 deletion in the mouse reveals non-reciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol Cell Biol 28:4675–4687
Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J (2006) The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev 20:3475–3486
Mu L, Berti L, Masserdotti G, Covic M, Michaelidis TM, Doberauer K, Merz K, Rehfeld F, Haslinger A, Wegner M, Sock E, Lefebvre V, Couillard-Despres S, Aigner L, Berninger B, Lie DC (2012) SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J Neurosci 32:3067–3080
Teddy JM, Kulesa PM (2004) In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development 131:6141–6151
Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, Whitington PM (2004) Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol 270:455–473
Koyanagi M, Urbich C, Chavakis E, Hoffmann J, Rupp S, Badorff C, Zeiher AM, Starzinski-Powitz A, Haendeler J, Dimmeler S (2005) Differentiation of circulating endothelial progenitor cells to a cardiomyogenic phenotype depends on E-cadherin. FEBS Lett 579:6060–6066
Luo Y, High FA, Epstein JA, Radice GL (2006) N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol 299:517–528
Komatsu K, Wakatsuki S, Yamada S, Yamamura K, Miyazaki J, Sehara-Fujisawa A (2007) Meltrin beta expressed in cardiac neural crest cells is required for ventricular septum formation of the heart. Dev Biol 303:82–92
Dy P, Penzo-Mendez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V (2008) The three SoxC proteins–Sox4, Sox11 and Sox12–exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res 36:3101–3117
Rochais F, Mesbah K, Kelly RG (2009) Signaling pathways controlling second heart field development. Circ Res 104:933–942
Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN (2007) Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development 134:1593–1604
Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM (2006) Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133:2419–2433
Sinha T, Wang B, Evans S, Wynshaw-Boris A, Wang J (2012) Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Dev Biol 370:135–144
Boogerd CJ, Wong LY, van den Boogaard M, Bakker ML, Tessadori F, Bakkers J, t Hoen PA, Moorman AF, Christoffels VM, Barnett P (2011) Sox4 mediates Tbx3 transcriptional regulation of the gap junction protein Cx43. Cell Mol Life Sci 68:3949–3961
Usui A, Mochizuki Y, Iida A, Miyauchi E, Satoh S, Sock E, Nakauchi H, Aburatani H, Murakami A, Wegner M, Watanabe S (2013) The early retinal progenitor-expressed gene Sox11 regulates the timing of the differentiation of retinal cells. Development 140:740–750
Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM (2006) SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience 143:501–514
Lin L, Lee VM, Wang Y, Lin JS, Sock E, Wegner M, Lei L (2011) Sox11 regulates survival and axonal growth of embryonic sensory neurons. Dev Dyn 240:52–64
Bergsland M, Ramskold D, Zaouter C, Klum S, Sandberg R, Muhr J (2011) Sequentially acting Sox transcription factors in neural lineage development. Genes Dev 25:2453–2464
Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM (2000) Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant. Cardiovasc Res 47:314–328
Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y (2003) Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am J Hum Genet 72:465–470
Brewer S, Jiang X, Donaldson S, Williams T, Sucov HM (2002) Requirement for AP-2α in cardiac outflow tract morphogenesis. Mech Dev 110:139–149
Seo S, Kume T (2006) Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol 296:421–436
Vincentz JW, Firulli BA, Lin A, Spicer DB, Howard MJ, Firulli AB (2013) Twist1 controls a cell-specification switch governing cell fate decisions within the cardiac neural crest. PLoS Genet 9:e1003405
Acknowledgments
Jürgen Behrens and Marina Kreutz are acknowledged for the gift of Ecadherin and Adam19 plasmids, respectively. This work was supported by a grant from the DFG to E.S. (So251/3-1) and from a National Health and Medical Research Council Australia Fellowship to RPH (573705).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, M.H., Harvey, R.P., Wegner, M. et al. Cardiac outflow tract development relies on the complex function of Sox4 and Sox11 in multiple cell types. Cell. Mol. Life Sci. 71, 2931–2945 (2014). https://doi.org/10.1007/s00018-013-1523-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1523-x