Abstract
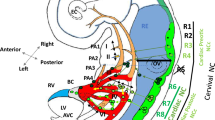
Congenital heart disease is the most common birth defects, affecting 1 % live births [1]. The cardiovascular system undergoes a series of morphogenetic events to form a heart and an aorta in fetuses. Formation of the heart and aorta requires migration, differentiation, and precise interactions among multiple cells from several embryonic origins [2]. Forkhead box2 (Foxc2) encodes a transcription factor and is expressed in mesodermal tissues, such as the pharyngeal artery, outflow tract endothelial/surrounding mesenchyme, bone, and kidney [3]. Simple knockout of Foxc2 in mouse causes an interrupted aortic arch, ventricular septal defect, cleft palate, and skeletal malformation [4]. The heart is made from primary and secondary heart field progenitors. The primary heart field gives rise to the left ventricle and atria, while the secondary heart field contributes mainly to the right ventricle and outflow tract [5] (Fig. 27.1).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Congenital heart disease is the most common birth defects, affecting 1 % live births [1]. The cardiovascular system undergoes a series of morphogenetic events to form a heart and an aorta in fetuses. Formation of the heart and aorta requires migration, differentiation, and precise interactions among multiple cells from several embryonic origins [2]. Forkhead box2 (Foxc2) encodes a transcription factor and is expressed in mesodermal tissues, such as the pharyngeal artery, outflow tract endothelial/surrounding mesenchyme, bone, and kidney [3]. Simple knockout of Foxc2 in mouse causes an interrupted aortic arch, ventricular septal defect, cleft palate, and skeletal malformation [4]. The heart is made from primary and secondary heart field progenitors. The primary heart field gives rise to the left ventricle and atria, while the secondary heart field contributes mainly to the right ventricle and outflow tract [5] (Fig. 27.1).
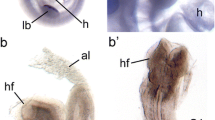
Aortic arch abnormalities in Foxc2 conditional knockout mice. (a) Normal aortic arch formation in control mice (Foxc2flox/flox). (b–d) Conditional knockout mice showed interrupted aortic arch (IAA) type B where part of the aorta between LCC and LSC is missing (arrow). LCC left common carotid artery, LSC left subclavian artery, aAo arch of the aorta, Pt pulmonary trunk, RCC right common carotid artery, RSC right subclavian artery, BC brachiocephalic artery
To explore the tissue-specific roles of Foxc2 in aortic arch remodeling, we generated mice carrying a floxed allele of Foxc2 (Foxc2flox) and crossed them with several Cre mice, including the primary heart field (Nkx2.5-Cre knock-in)-specific and secondary heart field (Islet1-Cre knock-in and Tbx1-Cre transgenic)-specific Cre lines. Surprisingly, conditional knockout (cKO) of Foxc2 in the primary heart field (Nkx2.5-Cre;Foxc2flox/flox) and secondary heart field (Islet1-Cre;Foxc2flox/flox and Tbx1-Cre;Foxc2flox/flox) resulted in an interrupted aortic arch and perinatal lethality in mice. X-gal staining and immunostaining with anti-Foxc2 antibody confirmed that Foxc2 expression in the aortic arch was intact but deleted in the outflow tract in these cKO embryos. These results indicate that the Foxc2 expression in the outflow tract, rather than direct role in the aortic arch, is crucial for the aortic arch remodeling. It assumed that Foxc2 in the outflow tract regulates aortic arch remodeling via secreted factors such as Fgf8, Fgf10, and other genes.
References
Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–8.
Olson EN, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–6.
Miura N, Wanaka A, et al. MFH-1, a new member of the fork head domain family, is expressed in developing mesenchyme. FEBS Lett. 1993;326:171–6.
Iida K, Miura N, et al. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development. 1997;124:4627–38.
Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18:210–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Uddin, M.K.M. et al. (2016). The Loss of Foxc2 Expression in the Outflow Tract Links the Interrupted Arch in the Conditional Foxc2 Knockout Mouse. In: Nakanishi, T., Markwald, R., Baldwin, H., Keller, B., Srivastava, D., Yamagishi, H. (eds) Etiology and Morphogenesis of Congenital Heart Disease. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54628-3_27
Download citation
DOI: https://doi.org/10.1007/978-4-431-54628-3_27
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54627-6
Online ISBN: 978-4-431-54628-3
eBook Packages: MedicineMedicine (R0)