Abstract
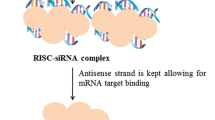
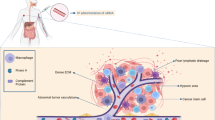
The identification of numerous deregulated signaling pathways on cancer cells and supportive stromal cells has revealed several molecular targets whose downregulation can elicit significant benefits for cancer treatment. In this respect, gene downregulation can be efficiently achieved by exploiting the RNA interference mechanism, particularly by the delivery of chemical synthesized small-interfering RNAs (siRNAs), which have the ability to mediate, in a specific manner, the degradation of any mRNA with complementary nucleotide sequence. However, several concerns regarding off-target effects and immune stimulation have been raised. Depending on their sequence, siRNAs can trigger an innate immune response, which might mediate undesirable side effects that ultimately compromise their clinical utility. This is a very relevant effect that will be discussed in the present manuscript. Moreover, the major drawback in the translation of siRNAs into the clinical practice is undoubtedly their inability to accumulate in tumor sites, particularly in organs other than the liver. In fact, upon systemic administration, owing to siRNAs physico-chemical features, they are rapidly cleared from the blood stream. Therefore, the development of a proper drug delivery system is of utmost importance. In this review, some of the latest advances on different nanotechnological platforms for siRNA delivery under clinical evaluation will be discussed. Along with this, targeting approaches towards cancer and/or endothelial cells will also be addressed, as these are some of the most promising strategies to enhance specific tumor accumulation while avoiding healthy tissues. Finally, clinical information on ongoing studies in patients with advanced solid tumors will be also provided.









Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62(1):10–29. doi:10.3322/caac.20138
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136(5):823–837. doi:10.1016/j.cell.2009.02.024
Farazi TA, Spitzer JI, Morozov P, Tuschl T (2011) miRNAs in human cancer. J Pathol 223(2):102–115. doi:10.1002/path.2806
Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA (2005) Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol 23(2):227–231. doi:10.1038/nbt1052
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411(6836):494–498. doi:10.1038/35078107
Aagaard L, Rossi JJ (2007) RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 59(2–3):75–86
de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J (2007) Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov 6(6):443–453
Bhindi R, Fahmy RG, Lowe HC, Chesterman CN, Dass CR, Cairns MJ, Saravolac EG, Sun LQ, Khachigian LM (2007) Brothers in arms: DNA enzymes, short interfering RNA, and the emerging wave of small-molecule nucleic acid-based gene-silencing strategies. Am J Pathol 171(4):1079–1088
Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, de Fougerolles T, Maraganore J (2010) A status report on RNAi therapeutics. Silence 1(1):14. doi:10.1186/1758-907X-1-14
Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS (2003) Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 21(6):635–637. doi:10.1038/nbt831
Tschuch C, Schulz A, Pscherer A, Werft W, Benner A, Hotz-Wagenblatt A, Barrionuevo LS, Lichter P, Mertens D (2008) Off-target effects of siRNA specific for GFP. BMC Mol Biol 9:60. doi:10.1186/1471-2199-9-60
Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A (2006) Off-target effects by siRNA can induce toxic phenotype. RNA 12(7):1188–1196. doi:10.1261/rna.28106
Mendonca LS, Firmino F, Moreira JN, Pedroso de Lima MC, Simoes S (2010) Transferrin receptor-targeted liposomes encapsulating anti-BCR-ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjug Chem 21(1):157–168. doi:10.1021/bc9004365
Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G (2005) Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 11(3):263–270. doi:10.1038/nm1191
Sioud M (2009) Deciphering the code of innate immunity recognition of siRNAs. Methods Mol Biol 487:41–59
Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I (2005) Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23(4):457–462. doi:10.1038/nbt1081
Sioud M (2005) Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol 348(5):1079–1090. doi:10.1016/j.jmb.2005.03.013
Sioud M (2006) Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: a central role for 2′-hydroxyl uridines in immune responses. Eur J Immunol 36(5):1222–1230. doi:10.1002/eji.200535708
Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR (2003) Activation of the interferon system by short-interfering RNAs. Nat Cell Biol 5(9):834–839. doi:10.1038/ncb1038
Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R (2003) Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet 34(3):263–264. doi:10.1038/ng1173
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004) Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303(5663):1526–1529. doi:10.1126/science.1093620
Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J (2008) Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452(7187):591–597. doi:10.1038/nature06765
Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J (2009) Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci USA 106(17):7137–7142. doi:10.1073/pnas.0812317106
Paone A, Galli R, Gabellini C, Lukashev D, Starace D, Gorlach A, De Cesaris P, Ziparo E, Del Bufalo D, Sitkovsky MV, Filippini A, Riccioli A (2010) Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia 12(7):539–549
Judge A, MacLachlan I (2008) Overcoming the innate immune response to small interfering RNA. Hum Gene Ther 19(2):111–124. doi:10.1089/hum.2007.179
Moreira JN, Santos A, Moura V, Pedroso de Lima MC, Simoes S (2008) Non-viral lipid-based nanoparticles for targeted cancer systemic gene silencing. J Nanosci Nanotechnol 8(5):2187–2204
Watts JK, Deleavey GF, Damha MJ (2008) Chemically modified siRNA: tools and applications. Drug Discov Today 13(19–20):842–855. doi:10.1016/j.drudis.2008.05.007
Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, Khvorova A, Linsley PS (2006) Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA 12(7):1197–1205. doi:10.1261/rna.30706
Chiu YL, Rana TM (2003) siRNA function in RNAi: a chemical modification analysis. RNA 9(9):1034–1048
Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B (2005) Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol 23(8):1002–1007. doi:10.1038/nbt1122
Morrissey DV, Blanchard K, Shaw L, Jensen K, Lockridge JA, Dickinson B, McSwiggen JA, Vargeese C, Bowman K, Shaffer CS, Polisky BA, Zinnen S (2005) Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology 41(6):1349–1356. doi:10.1002/hep.20702
Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I (2006) RNAi-mediated gene silencing in non-human primates. Nature 441(7089):111–114. doi:10.1038/nature04688
Judge AD, Bola G, Lee AC, MacLachlan I (2006) Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther 13(3):494–505. doi:10.1016/j.ymthe.2005.11.002
Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C (2006) Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol 36(12):3256–3267. doi:10.1002/eji.200636617
Sioud M, Furset G, Cekaite L (2007) Suppression of immunostimulatory siRNA-driven innate immune activation by 2′-modified RNAs. Biochem Biophys Res Commun 361(1):122–126. doi:10.1016/j.bbrc.2007.06.177
Robbins M, Judge A, Liang L, McClintock K, Yaworski E, MacLachlan I (2007) 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther 15(9):1663–1669. doi:10.1038/sj.mt.6300240
Hamm S, Latz E, Hangel D, Muller T, Yu P, Golenbock D, Sparwasser T, Wagner H, Bauer S (2010) Alternating 2′-O-ribose methylation is a universal approach for generating non-stimulatory siRNA by acting as TLR7 antagonist. Immunobiology 215(7):559–569. doi:10.1016/j.imbio.2009.09.003
Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, McClintock K, MacLachlan I (2008) Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther 19(10):991–999. doi:10.1089/hum.2008.131
Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J (2004) Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA 101(23):8676–8681. doi:10.1073/pnas.0402486101
Tompkins SM, Lo CY, Tumpey TM, Epstein SL (2004) Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA 101(23):8682–8686. doi:10.1073/pnas.0402630101
Furset G, Sioud M (2007) Design of bifunctional siRNAs: combining immunostimulation and gene-silencing in one single siRNA molecule. Biochem Biophys Res Commun 352(3):642–649. doi:10.1016/j.bbrc.2006.11.059
Iversen PO, Semaeva E, Sorensen DR, Wiig H, Sioud M (2009) Dendritic cells loaded with tumor antigens and a dual immunostimulatory and anti-interleukin 10-specific small interference RNA prime t lymphocytes against leukemic cells. Transl Oncol 2(4):242–246
Khairuddin N, Gantier MP, Blake SJ, Wu SY, Behlke MA, Williams BR, McMillan NA (2011) siRNA-induced immunostimulation through TLR7 promotes antitumoral activity against HPV-driven tumors in vivo. Immunol Cell Biol. doi:10.1038/icb.2011.19
Lu PY, Woodle MC (2008) Delivering small interfering RNA for novel therapeutics. Methods Mol Biol 437:93–107. doi:10.1007/978-1-59745-210-6_3
Castanotto D, Rossi JJ (2009) The promises and pitfalls of RNA-interference-based therapeutics. Nature 457(7228):426–433. doi:10.1038/nature07758
Bumcrot D, Manoharan M, Koteliansky V, Sah DW (2006) RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol 2(12):711–719. doi:10.1038/nchembio839
Gomes-da-Silva LC, Fonseca NA, Moura V, Pedroso de Lima MC, Simoes S, Moreira JN (2012) Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc Chem Res 45(7):1163–1171. doi:10.1021/ar300048p
Rossi JJ (2008) Expression strategies for short hairpin RNA interference triggers. Hum Gene Ther 19(4):313–317
Kim DH, Rossi JJ (2007) Strategies for silencing human disease using RNA interference. Nat Rev Genet 8(3):173–184. doi:10.1038/nrg2006
Knop K, Hoogenboom R, Fischer D, Schubert US (2010) Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl 49(36):6288–6308. doi:10.1002/anie.200902672
Heyes J, Palmer L, Bremner K, MacLachlan I (2005) Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release 107(2):276–287. doi:10.1016/j.jconrel.2005.06.014
Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ (2010) Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 28(2):172–176. doi:10.1038/nbt.1602
Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I (2005) A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm Res 22(3):362–372
Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee AC, Judge A, Jeffs LB, MacLachlan I (2006) Postexposure protection of guinea pigs against a lethal Ebola virus challenge is conferred by RNA interference. J Infect Dis 193(12):1650–1657. doi:10.1086/504267
Semple SC, Klimuk SK, Harasym TO, Dos Santos N, Ansell SM, Wong KF, Maurer N, Stark H, Cullis PR, Hope MJ, Scherrer P (2001) Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta 1510(1–2):152–166 (pii:S0005-2736(00)00343-6)
Maurer N, Wong KF, Stark H, Louie L, McIntosh D, Wong T, Scherrer P, Semple SC, Cullis PR (2001) Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys J 80(5):2310–2326. doi:10.1016/S0006-3495(01)76202-9
Ambegia E, Ansell S, Cullis P, Heyes J, Palmer L, MacLachlan I (2005) Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim Biophys Acta 1669(2):155–163. doi:10.1016/j.bbamem.2005.02.001
Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I (2009) Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest 119(3):661–673. doi:10.1172/JCI37515
Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, Hope MJ, Scherrer P, Cullis PR (1999) Stabilized plasmid-lipid particles: construction and characterization. Gene Ther 6(2):271–281. doi:10.1038/sj.gt.3300821
Reddy LH, Couvreur P (2011) Nanotechnology for therapy and imaging of liver diseases. J Hepatol 55(6):1461–1466. doi:10.1016/j.jhep.2011.05.039
Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA (2010) Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 18(7):1357–1364. doi:10.1038/mt.2010.85
Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, Maclachlan I (2010) Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet 375(9729):1896–1905. doi:10.1016/S0140-6736(10)60357-1
Lin X, Li L, Wang R, Wilcox D, Zhao X, Song J, Huang X, Hansen TM, Dande P, Wada C, Hubbard RD, Kohlbrenner WM, Fesik SW, Shen Y (2011) A robust in vivo positive-readout system for monitoring siRNA delivery to xenograft tumors. RNA 17(4):603–612. doi:10.1261/rna.2546011
Li L, Wang R, Wilcox D, Zhao X, Song J, Lin X, Kohlbrenner WM, Fesik SW, Shen Y (2011) Tumor vasculature is a key determinant for the efficiency of nanoparticle-mediated siRNA delivery. Gene Ther. doi:10.1038/gt.2011.146
Jain RK (1994) Barriers to drug delivery in solid tumors. Sci Am 271(1):58–65
Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 7(11):653–664. doi:10.1038/nrclinonc.2010.139
Jang SH, Wientjes MG, Lu D, Au JL (2003) Drug delivery and transport to solid tumors. Pharm Res 20(9):1337–1350
Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN (2010) Hypoxia inducible factors in cancer stem cells. Br J Cancer 102(5):789–795. doi:10.1038/sj.bjc.6605551
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10(6):417–427. doi:10.1038/nrd3455
Gomes CP, Gomes-da-Silva LC, Ramalho JS, de Lima MC, Simoes S, Moreira JN (2013) Impact Of Plk-1 Silencing On Endothelial Cells And Cancer Cells Of Diverse Histological Origin. Curr Gene Ther 13(3):189–201 (pii:CGT-EPUB-20130325-3)
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63(3):136–151. doi:10.1016/j.addr.2010.04.009
Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG (2010) Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA 107(5):1864–1869. doi:10.1073/pnas.0910603106
Liu Y, Huang L (2010) Designer lipids advance systemic siRNA delivery. Mol Ther 18(4):669–670. doi:10.1038/mt.2010.39
Bellocq NC, Pun SH, Jensen GS, Davis ME (2003) Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem 14(6):1122–1132. doi:10.1021/bc034125f
Davis ME (2009) The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 6(3):659–668. doi:10.1021/mp900015y
Heidel JD, Liu JY, Yen Y, Zhou B, Heale BS, Rossi JJ, Bartlett DW, Davis ME (2007) Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin Cancer Res 13(7):2207–2215. doi:10.1158/1078-0432.CCR-06-2218
Bartlett DW, Davis ME (2008) Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol Bioeng 99(4):975–985. doi:10.1002/bit.21668
Rahman MA, Amin AR, Wang X, Zuckerman JE, Choi CH, Zhou B, Wang D, Nannapaneni S, Koenig L, Chen Z, Chen ZG, Yen Y, Davis ME, Shin DM (2012) Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J Control Release 159(3):384–392. doi:10.1016/j.jconrel.2012.01.045
Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J (2006) A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther 13(16):1222–1234. doi:10.1038/sj.gt.3302777
Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, Loffler K, Fechtner M, Rohl T, Fisch G, Dames S, Arnold W, Giese K, Klippel A, Kaufmann J (2006) RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther 13(18):1360–1370. doi:10.1038/sj.gt.3302778
Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Roder N, Loffler K, Lange C, Fechtner M, Mopert K, Fisch G, Dames S, Arnold W, Jochims K, Giese K, Wiedenmann B, Scholz A, Kaufmann J (2008) Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res 68(23):9788–9798. doi:10.1158/0008-5472.CAN-08-2428
Santel A, Aleku M, Roder N, Mopert K, Durieux B, Janke O, Keil O, Endruschat J, Dames S, Lange C, Eisermann M, Loffler K, Fechtner M, Fisch G, Vank C, Schaeper U, Giese K, Kaufmann J (2010) Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res 16(22):5469–5480. doi:10.1158/1078-0432.CCR-10-1994
Mopert K, Loffler K, Roder N, Kaufmann J, Santel A (2012) Depletion of protein kinase N3 (PKN3) impairs actin and adherens junctions dynamics and attenuates endothelial cell activation. Eur J Cell Biol 91(9):694–705. doi:10.1016/j.ejcb.2012.03.010
Gutierrez-Puente Y, Tari AM, Stephens C, Rosenblum M, Guerra RT, Lopez-Berestein G (1999) Safety, pharmacokinetics, and tissue distribution of liposomal P-ethoxy antisense oligonucleotides targeted to Bcl-2. J Pharmacol Exp Ther 291(2):865–869
Landen CN Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK (2005) Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res 65(15):6910–6918. doi:10.1158/0008-5472.CAN-05-0530
Landen CN, Merritt WM, Mangala LS, Sanguino AM, Bucana C, Lu C, Lin YG, Han LY, Kamat AA, Schmandt R, Coleman RL, Gershenson DM, Lopez-Berestein G, Sood AK (2006) Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther 5(12):1708–1713 (pii:3468)
Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, Mangala LS, Lu Y, Baggerly KA, Danes CG, Nick AM, Halder J, Kim HS, Vivas-Mejia P, Landen CN, Lopez-Berestein G, Coleman RL, Sood AK (2009) Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther 8(11):1027–1034 (pii:8523)
Gaspar R, Duncan R (2009) Polymeric carriers: preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv Drug Deliv Rev 61(13):1220–1231. doi:10.1016/j.addr.2009.06.003
Karmali PP, Chaudhuri A (2007) Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med Res Rev 27(5):696–722. doi:10.1002/med.20090
Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC (2000) Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther 292(3):1071–1079
Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H (2005) Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release 105(3):305–317. doi:10.1016/j.jconrel.2005.04.003
Ishida T, Ichihara M, Wang X, Kiwada H (2006) Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release 115(3):243–250. doi:10.1016/j.jconrel.2006.08.001
Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H (2006) Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release 112(1):15–25. doi:10.1016/j.jconrel.2006.01.005
Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N (2010) T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm 392(1–2):218–223. doi:10.1016/j.ijpharm.2010.03.022
Judge A, McClintock K, Phelps JR, Maclachlan I (2006) Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther 13(2):328–337. doi:10.1016/j.ymthe.2005.09.014
Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ (2005) Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J Pharmacol Exp Ther 312(3):1020–1026. doi:10.1124/jpet.104.078113
Tagami T, Nakamura K, Shimizu T, Ishida T, Kiwada H (2009) Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. J Control Release 137(3):234–240. doi:10.1016/j.jconrel.2009.04.006
Tagami T, Uehara Y, Moriyoshi N, Ishida T, Kiwada H (2011) Anti-PEG IgM production by siRNA encapsulated in a PEGylated lipid nanocarrier is dependent on the sequence of the siRNA. J Control Release 151(2):149–154. doi:10.1016/j.jconrel.2010.12.013
Barros SA, Gollob JA (2012) Safety profile of RNAi nanomedicines. Adv Drug Deliv Rev 64(15):1730–1737. doi:10.1016/j.addr.2012.06.007
Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ (2005) Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res 65(19):8984–8992. doi:10.1158/0008-5472.CAN-05-0565
Allen TM (2002) Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2(10):750–763. doi:10.1038/nrc903
Santos AO, da Silva LC, Bimbo LM, de Lima MC, Simoes S, Moreira JN (2010) Design of peptide-targeted liposomes containing nucleic acids. Biochim Biophys Acta 1798(3):433–441. doi:10.1016/j.bbamem.2009.12.001
Woll PJ, Rozengurt E (1990) A neuropeptide antagonist that inhibits the growth of small cell lung cancer in vitro. Cancer Res 50(13):3968–3973
Mishra S, Webster P, Davis ME (2004) PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol 83(3):97–111
Li SD, Chen YC, Hackett MJ, Huang L (2008) Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther 16(1):163–169. doi:10.1038/sj.mt.6300323
Li SD, Chono S, Huang L (2008) Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther 16(5):942–946. doi:10.1038/mt.2008.51
Gao J, Liu W, Xia Y, Li W, Sun J, Chen H, Li B, Zhang D, Qian W, Meng Y, Deng L, Wang H, Chen J, Guo Y (2011) The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials 32(13):3459–3470. doi:10.1016/j.biomaterials.2011.01.034
Gao J, Yu Y, Zhang Y, Song J, Chen H, Li W, Qian W, Deng L, Kou G, Chen J, Guo Y (2012) EGFR-specific PEGylated immunoliposomes for active siRNA delivery in hepatocellular carcinoma. Biomaterials 33(1):270–282. doi:10.1016/j.biomaterials.2011.09.035
Mendonca LS, Moreira JN, de Lima MC, Simoes S (2010) Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol Bioeng 107(5):884–893. doi:10.1002/bit.22858
Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ (2006) Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release 112(2):199–207. doi:10.1016/j.jconrel.2006.02.011
Chiu SJ, Marcucci G, Lee RJ (2006) Efficient delivery of an antisense oligodeoxyribonucleotide formulated in folate receptor-targeted liposomes. Anticancer Res 26(2A):1049–1056
Yang L, Li J, Zhou W, Yuan X, Li S (2004) Targeted delivery of antisense oligodeoxynucleotides to folate receptor-overexpressing tumor cells. J Control Release 95(2):321–331. doi:10.1016/j.jconrel.2003.11.021
Feng C, Wang T, Tang R, Wang J, Long H, Gao X, Tang S (2010) Silencing of the MYCN gene by siRNA delivered by folate receptor-targeted liposomes in LA-N-5 cells. Pediatr Surg Int 26(12):1185–1191. doi:10.1007/s00383-010-2703-5
Cardoso AL, Simoes S, de Almeida LP, Pelisek J, Culmsee C, Wagner E, Pedroso de Lima MC (2007) siRNA delivery by a transferrin-associated lipid-based vector: a non-viral strategy to mediate gene silencing. J Gene Med 9(3):170–183. doi:10.1002/jgm.1006
Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464(7291):1067–1070. doi:10.1038/nature08956
Lu W, Zhang G, Zhang R, Flores LG 2nd, Huang Q, Gelovani JG, Li C (2010) Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res 70(8):3177–3188. doi:10.1158/0008-5472.CAN-09-3379
Sapra P, Allen TM (2003) Ligand-targeted liposomal anticancer drugs. Prog Lipid Res 42(5):439–462 (pii:S0163782703000328)
Sapra P, Tyagi P, Allen TM (2005) Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv 2(4):369–381
Zhu Q, Feng C, Liao W, Zhang Y, Tang S (2013) Target delivery of MYCN siRNA by folate-nanoliposomes delivery system in a metastatic neuroblastoma model. Cancer Cell Int 13(1):65. doi:10.1186/1475-2867-13-65
da Cruz MT, Simoes S, Pires PP, Nir S, de Lima MC (2001) Kinetic analysis of the initial steps involved in lipoplex–cell interactions: effect of various factors that influence transfection activity. Biochim Biophys Acta 1510(1–2):136–151 (pii:S0005273600003424)
Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME (2007) Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA 104(39):15549–15554. doi:10.1073/pnas.0707461104
Iyer AK, Khaled G, Fang J, Maeda H (2006) Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 11(17–18):812–818. doi:10.1016/j.drudis.2006.07.005
Li SD, Huang L (2008) Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 5(4):496–504. doi:10.1021/mp800049w
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29(6 Suppl 16):15–18. doi:10.1053/sonc.2002.37263
Abdollahi A, Folkman J (2010) Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat 13(1–2):16–28. doi:10.1016/j.drup.2009.12.001
Simone E, Ding BS, Muzykantov V (2009) Targeted delivery of therapeutics to endothelium. Cell Tissue Res 335(1):283–300. doi:10.1007/s00441-008-0676-7
Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR (2006) Advanced drug delivery systems that target the vascular endothelium. Mol Interv 6(2):98–112. doi:10.1124/mi.6.2.7
Hajitou A, Pasqualini R, Arap W (2006) Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc Med 16(3):80–88. doi:10.1016/j.tcm.2006.01.003
Chen Y, Wu JJ, Huang L (2010) Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol Ther 18(4):828–834. doi:10.1038/mt.2009.291
Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E (2002) A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci USA 99(11):7444–7449. doi:10.1073/pnas.062189599
Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E (2003) Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol 163(4):871–878. doi:10.1083/jcb.200304132
Gomes-da-Silva LC, Santos AO, Bimbo LM, Moura V, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN (2012) Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int J Pharm 434(1–2):9–19. doi:10.1016/j.ijpharm.2012.05.018
Gomes-da-Silva LC, Fernandez Y, Abasolo I, Schwartz S, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN (2013) Efficient intracellular delivery of siRNA with a safe multitargeted lipid-based nanoplatform. Nanomedicine (Lond). doi:10.2217/nnm.12.174
Gomes-da-Silva LC, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN (2013) Impact of anti-PLK1 siRNA-containing F3-targeted liposomes on the viability of both cancer and endothelial cells. Eur J Pharm Biopharm. doi:10.1016/j.ejpb.2013.04.007
Moura V, Lacerda M, Figueiredo P, Corvo ML, Cruz ME, Soares R, de Lima MC, Simoes S, Moreira JN (2012) Targeted and intracellular triggered delivery of therapeutics to cancer cells and the tumor microenvironment: impact on the treatment of breast cancer. Breast Cancer Res Treat 133(1):61–73. doi:10.1007/s10549-011-1688-7
Strumberg D, Schultheis B, Traugott U, Vank C, Santel A, Keil O, Giese K, Kaufmann J, Drevs J (2012) Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther 50(1):76–78 (pii:9341)
Acknowledgments
The authors would like to acknowledge Nuno Fonseca for his help with Fig. 1. The work performed by the authors was supported by the Portugal–Spain capacitation program in Nanoscience and Nanotechnology (ref.: NANO/NMed-AT/0042/2007) and grant PEst-C/SAU/LA0001/2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomes-da-Silva, L.C., Simões, S. & Moreira, J.N. Challenging the future of siRNA therapeutics against cancer: the crucial role of nanotechnology. Cell. Mol. Life Sci. 71, 1417–1438 (2014). https://doi.org/10.1007/s00018-013-1502-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1502-2