Abstract
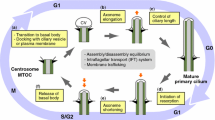
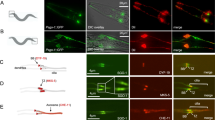
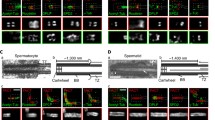
Nucleotide-binding proteins Nubp1 and Nubp2 are MRP/MinD-type P-loop NTPases with sequence similarity to bacterial division site-determining proteins and are conserved, essential proteins throughout the Eukaryotes. They have been implicated, together with their interacting minus-end directed motor protein KIFC5A, in the regulation of centriole duplication in mammalian cells. Here we show that Nubp1 and Nubp2 are integral components of centrioles throughout the cell cycle, recruited independently of KIFC5A. We further demonstrate their localization at the basal body of the primary cilium in quiescent vertebrate cells or invertebrate sensory cilia, as well as in the motile cilia of mouse cells and in the flagella of Chlamydomonas. RNAi-mediated silencing of nubp-1 in C. elegans causes the formation of morphologically aberrant and additional cilia in sensory neurons. Correspondingly, downregulation of Nubp1 or Nubp2 in mouse quiescent NIH 3T3 cells markedly increases the number of ciliated cells, while knockdown of KIFC5A dramatically reduces ciliogenesis. Simultaneous double silencing of Nubp1 + KIFC5A restores the percentage of ciliated cells to control levels. We document the normal ciliary recruitment, during these silencing regimes, of basal body proteins critical for ciliogenesis, namely CP110, CEP290, cenexin, Chibby, AurA, Rab8, and BBS7. Interestingly, we uncover novel interactions of Nubp1 with several members of the CCT/TRiC molecular chaperone complex, which we find enriched at the basal body and recruited independently of the Nubps or KIFC5A. Our combined results for Nubp1, Nubp2, and KIFC5A and their striking effects on cilium formation suggest a central regulatory role for these proteins, likely involving CCT/TRiC chaperone activity, in ciliogenesis.








Similar content being viewed by others
References
Dawe HR, Farr H, Gull K (2007) Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci 120(Pt 1):7–15
Pedersen LB, Veland IR, Schroder JM, Christensen ST (2008) Assembly of primary cilia. Dev Dyn 237(8):1993–2006
Gerdes JM, Davis EE, Katsanis N (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137(1):32–45
Satir P, Pedersen LB, Christensen ST (2010) The primary cilium at a glance. J Cell Sci 123(Pt 4):499–503
Seeley ES, Nachury MV (2010) The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci 123(Pt 4):511–518
Christensen ST, Pedersen LB, Schneider L, Satir P (2007) Sensory cilia and integration of signal transduction in human health and disease. Traffic 8(2):97–109
Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH Jr, Dlugosz AA, Reiter JF (2009) Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 15(9):1055–1061
Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11(5):331–344
Wallingford JB, Mitchell B (2011) Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 25(3):201–213
Hoyer-Fender S (2010) Centriole maturation and transformation to basal body. Semin Cell Dev Biol 21(2):142–147
Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T (2005) The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol 25(10):4129–4137
Anderson RG (1972) The three-dimensional structure of the basal body from the rhesus monkey oviduct. J Cell Biol 54(2):246–265
Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL (2001) Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol 11(20):1586–1590
Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ (2010) A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329(5990):436–439
Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ (2010) Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol 12(7):703–710
Spektor A, Tsang WY, Khoo D, Dynlacht BD (2007) Cep97 and CP110 suppress a cilia assembly program. Cell 130(4):678–690
Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD (2008) CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell 15(2):187–197
Kim J, Krishnaswami SR, Gleeson JG (2008) CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet 17(23):3796–3805
Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129(7):1351–1363
Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J (2012) The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol 14(9):950–957
Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM (2005) Functional coordination of intraflagellar transport motors. Nature 436(7050):583–587
Scholey JM, Anderson KV (2006) Intraflagellar transport and cilium-based signaling. Cell 125(3):439–442
Pedersen LB, Rosenbaum JL (2008) Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85:23–61
Silverman MA, Leroux MR (2009) Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol 19(7):306–316
Karsenti E, Vernos I (2001) The mitotic spindle: a self-made machine. Science 294(5542):543–547
Johnson KA, Rosenbaum JL (1992) Polarity of flagellar assembly in Chlamydomonas. J Cell Biol 119(6):1605–1611
Marshall WF, Rosenbaum JL (2001) Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol 155(3):405–414
Akhmanova A, Steinmetz MO (2008) Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 9(4):309–322
Schuyler SC, Pellman D (2001) Microtubule “plus-end-tracking proteins”: the end is just the beginning. Cell 105(4):421–424
Vaughan KT (2005) TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. J Cell Biol 171(2):197–200
Schrøder JM, Schneider L, Christensen ST, Pedersen LB (2007) EB1 is required for primary cilia assembly in fibroblasts. Curr Biol 17(13):1134–1139
Schrøder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, Pedersen LB (2011) EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci 124(Pt 15):2539–2551
Horwich AL, Fenton WA, Chapman E, Farr GW (2007) Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol 23:115–145
Kabir MA, Uddin W, Narayanan A, Reddy PK, Jairajpuri MA, Sherman F, Ahmad Z (2011) Functional subunits of eukaryotic chaperonin CCT/TRiC in protein folding. J Amino Acids 2011:843206
Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H (1992) TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature 358(6383):245–248
Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ (1992) A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell 69(6):1043–1050
Melki R, Vainberg IE, Chow RL, Cowan NJ (1993) Chaperonin-mediated folding of vertebrate actin-related protein and gamma-tubulin. J Cell Biol 122(6):1301–1310
Bloch MA, Johnson KA (1995) Identification of a molecular chaperone in the eukaryotic flagellum and its localization to the site of microtubule assembly. J Cell Sci 108(Pt 11):3541–3545
Kim JC, Ou YY, Badano JL, Esmail MA, Leitch CC, Fiedrich E, Beales PL, Archibald JM, Katsanis N, Rattner JB, Leroux MR (2005) MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet–Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci 118(Pt 5):1007–1020
Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, McKay SJ, Huang P, Swoboda P, Jones SJ, Marra MA, Baillie DL, Moerman DG, Shaham S, Leroux MR (2005) Functional genomics of the cilium, a sensory organelle. Curr Biol 15(10):935–941
Seixas C, Cruto T, Tavares A, Gaertig J, Soares H (2010) CCTalpha and CCTdelta chaperonin subunits are essential and required for cilia assembly and maintenance in Tetrahymena. PLoS One 5(5):e10704
Seo S, Baye LM, Schulz NP, Beck JS, Zhang Q, Slusarski DC, Sheffield VC (2010) BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci USA 107(4):1488–1493
Plotnikova OV, Golemis EA, Pugacheva EN (2008) Cell cycle-dependent ciliogenesis and cancer. Cancer Res 68(7):2058–2061
Godinho SA, Kwon M, Pellman D (2009) Centrosomes and cancer: how cancer cells divide with too many centrosomes. Cancer Metastasis Rev 28(1–2):85–98. doi:10.1007/s10555-008-9163-6
Hatch E, Stearns T (2010) The life cycle of centrioles. Cold Spring Harb Symp Quant Biol 75:425–431
Mahjoub MR, Stearns T (2012) Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Curr Biol 22(17):1628–1634
Shahrestanifar M, Saha DP, Scala LA, Basu A, Howells RD (1994) Cloning of a human cDNA encoding a putative nucleotide-binding protein related to Escherichia coli MinD. Gene 147(2):281–285
Nakashima H, Grahovac MJ, Mazzarella R, Fujiwara H, Kitchen JR, Threat TA, Ko MS (1999) Two novel mouse genes–Nubp2, mapped to the t-complex on chromosome 17, and Nubp1, mapped to chromosome 16–establish a new gene family of nucleotide-binding proteins in eukaryotes. Genomics 60(2):152–160
Christodoulou A, Lederer CW, Surrey T, Vernos I, Santama N (2006) Motor protein KIFC5A interacts with Nubp1 and Nubp2, and is implicated in the regulation of centrosome duplication. J Cell Sci 119(Pt 10):2035–2047
Leipe DD, Wolf YI, Koonin EV, Aravind L (2002) Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol 317(1):41–72
Vitale G, Fabre E, Hurt EC (1996) NBP35 encodes an essential and evolutionary conserved protein in Saccharomyces cerevisiae with homology to a superfamily of bacterial ATPases. Gene 178(1–2):97–106
Hausmann A, Aguilar Netz DJ, Balk J, Pierik AJ, Muhlenhoff U, Lill R (2005) The eukaryotic P loop NTPase Nbp35: an essential component of the cytosolic and nuclear iron-sulfur protein assembly machinery. Proc Natl Acad Sci USA 102(9):3266–3271
Netz DJ, Pierik AJ, Stumpfig M, Muhlenhoff U, Lill R (2007) The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol. Nat Chem Biol 3(5):278–286
Stehling O, Netz DJ, Niggemeyer B, Rosser R, Eisenstein RS, Puccio H, Pierik AJ, Lill R (2008) Human Nbp35 is essential for both cytosolic iron-sulfur protein assembly and iron homeostasis. Mol Cell Biol 28(17):5517–5528
Netz DJ, Pierik AJ, Stumpfig M, Bill E, Sharma AK, Pallesen LJ, Walden WE, Lill R (2012) A bridging [4Fe-4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron-sulfur protein maturation. J Biol Chem 287(15):12365–12378
Pedersen LB, Geimer S, Sloboda RD, Rosenbaum JL (2003) The microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr Biol 13(22):1969–1974
Pedersen LB, Rompolas P, Christensen ST, Rosenbaum JL, King SM (2007) The lissencephaly protein Lis1 is present in motile mammalian cilia and requires outer arm dynein for targeting to Chlamydomonas flagella. J Cell Sci 120(Pt 5):858–867
D’Angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, Dynlacht B, Pagano M (2010) SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466(7302):138–142
Rieckher M, Kourtis N, Pasparaki A, Tavernarakis N (2009) Transgenesis in Caenorhabditis elegans. Methods Mol Biol 561:21–39
Troemel ER, Kimmel BE, Bargmann CI (1997) Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91(2):161–169
Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2 (1):RESEARCH0002
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA 54(6):1665–1669
Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL (1998) Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141(4):993–1008
Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR (2008) ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J Cell Biol 183(2):313–322
Pedersen LB, Geimer S, Rosenbaum JL (2006) Dissecting the molecular mechanisms of intraflagellar transport in Chlamydomonas. Curr Biol 16(5):450–459. doi:10.1016/j.cub.2006.02.020
Jakobsen L, Vanselow K, Skogs M, Toyoda Y, Lundberg E, Poser I, Falkenby LG, Bennetzen M, Westendorf J, Nigg EA, Uhlen M, Hyman AA, Andersen JS (2011) Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. EMBO J 30(8):1520–1535
Pazour GJ, Agrin N, Leszyk J, Witman GB (2005) Proteomic analysis of a eukaryotic cilium. J Cell Biol 170:103–113
Dymek EE, Goduti D, Kramer T, Smith EF (2006) A kinesin-like calmodulin-binding protein in Chlamydomonas: evidence for a role in cell division and flagellar functions. J Cell Sci 119(Pt 15):3107–3116
Bargmann CI, Mori I (1997) Chemotaxis and thermotaxis. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans II, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Driscoll M, Kaplan J (1997) Mechanotransduction. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans II, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Riddle DL, Albert PS (1997) Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans II, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Hilliard MA, Bargmann CI, Bazzicalupo P (2002) C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol 12(9):730–734
Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT (2003) Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature 422(6934):905–909
Steere N, Chae V, Burke M, Li FQ, Takemaru K, Kuriyama R (2012) A Wnt/beta-catenin pathway antagonist Chibby binds cenexin at the distal end of mother centrioles and functions in primary cilia formation. PLoS One 7(7):e41077
Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD (2002) CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell 3(3):339–350
Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA (2007) Plk4-induced centriole biogenesis in human cells. Dev Cell 13:190–202
Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129(6):1201–1213
Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA (2007) Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178(3):363–369
Peränen J (2011) Rab8 GTPase as a regulator of cell shape. Cytoskeleton (Hoboken) 68(10):527–539
Salisbury JL, Suino KM, Busby R, Springett M (2002) Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol 12(15):1287–1292
Kubota H, Hynes G, Willison K (1995) The chaperonin containing t-complex polypeptide 1 (TCP-1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem 230(1):3–16
Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB (1993) The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci USA 90(20):9422–9426
Dunn AY, Melville MW, Frydman J (2001) Review: cellular substrates of the eukaryotic chaperonin TRiC/CCT. J Struct Biol 135(2):176–184
Spiess C, Meyer AS, Reissmann S, Frydman J (2004) Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol 14(11):598–604
Dekker C, Stirling PC, McCormack EA, Filmore H, Paul A, Brost RL, Costanzo M, Boone C, Leroux MR, Willison KR (2008) The interaction network of the chaperonin CCT. EMBO J 27(13):1827–1839
Camasses A, Bogdanova A, Shevchenko A, Zachariae W (2003) The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol Cell 12(1):87–100
Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB (2009) The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol 187(7):1117–1132
Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC (2012) Intrinsic protein–protein interaction-mediated and chaperonin-assisted sequential assembly of stable Bardet–Biedl syndrome protein complex, the BBSome. J Biol Chem 287(24):20625–20635
Okuno T, Yamabayashi H, Kogure K (2010) Comparison of intracellular localization of Nubp1 and Nubp2 using GFP fusion proteins. Mol Biol Rep 37(3):1165–1168
Thulasiraman V, Yang CF, Frydman J (1999) In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J 18(1):85–95
Melki R, Cowan NJ (1994) Facilitated folding of actins and tubulins occurs via a nucleotide-dependent interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol Cell Biol 14(5):2895–2904
Melki R (2001) Review: nucleotide-dependent conformational changes of the chaperonin containing TCP-1. J Struct Biol 135(2):170–175
Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ (1995) Quasi-native chaperonin-bound intermediates in facilitated protein folding. J Biol Chem 270(41):23910–23913
Guenther MG, Yu J, Kao GD, Yen TJ, Lazar MA (2002) Assembly of the SMRT-histone deacetylase 3 repression complex requires the TCP-1 ring complex. Genes Dev 16(24):3130–3135
Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA (2009) Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci 122(Pt 15):2716–2726
Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464(7291):1048–1051
Ioannou A, Santama N, Skourides P (2013) Xenopus laevis nucleotide-binding protein 1 (xNubp1) is important for convergent extension movements and controls ciliogenesis via regulation of the actin cytoskeleton. Dev Biol. doi:10.1016/j.ydbio.2013.05.004. [Epub ahead of print]
Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK (2011) Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell 22(6):806–816
Kobayashi T, Dynlacht BD (2011) Regulating the transition from centriole to basal body. J Cell Biol 193(3):435–444
Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J (2009) A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci USA 106(12):4713–4718
Schnatwin C, Niswander L (2012) Nubp1 is required for lung branching morphogenesis and distal progenitor cell survival in mice. PLoS One 7(9):e44871
Perdiz D, Mackeh R, Poüs C, Baillet A (2011) The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signal 23(5):763–771
Piperno G, LeDizet M, Chang XJ (1987) Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104(2):289–302
Acknowledgments
We warmly thank our colleagues for kindly providing antibodies: Profs. Ryoko Kuriyama (University of Minnesota), Anand Swaroop (NIH, Bethesda), Isabelle Vernos (CRG, Barcelona), Johan Peränen (University of Helsinki), Joel Rosenbaum (Yale University) and Elizabeth Smith (Dartmouth College, NH). We are indebted to Ioanna Georgiou and Maria Christoforou (University of Cyprus) for cell-cycle analysis of Nubp1 and to Elena Panayiotou-Worth (Cyprus Institute of Neurology and Genetics) for providing us with mouse tracheal sections. This work was funded by the Research Promotion Foundation of Cyprus and Structural Funds from the EU (grant DIDAKTOR/DISEK/0308-05 to N.S.) and the Danish Natural Science Research Council (grants 09-070398 and 10-085373 to L.B.P.). E.K. received a FEBS short-term fellowship to visit the laboratory of N.T. for work with C. elegans.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Kypri, A. Christodoulou, and G. Maimaris contributed equally to the experimental work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2013_1401_MOESM1_ESM.eps
Supplementary material 1 Detection of Nubp1 and Nubp2 in mouse NIH 3T3 cells. A–B. WB analysis of total protein extracts from NIH 3T3 fibroblasts, using affinity-purified rabbit anti-Nubp1 ab (A) and rat anti-Nubp2 ab (B), reveals unique bands consistent with the predicted Mr for Nubp1 (34.085 × 103) and Nubp2 (29.518 × 103). (EPS 1040 kb)
18_2013_1401_MOESM2_ESM.eps
Supplementary material 2 A. Localization of Nubp2 during the sub-phases of the mitosis in NIH 3T3 fibroblasts. NIH 3T3 cells were labeled with an ab against α-tubulin (red) and an ab against Nubp2 (green). DNA was visualized with Hoechst (blue). Scale bars 10 μm. B. Nubp1 and Nubp2 are co-localized. Example of co-localization of Nubp2 (red) and Nubp1 (green) in prometaphase. DNA was visualized with Hoechst (blue). Scale bars 10 μm. (EPS 17527 kb)
18_2013_1401_MOESM3_ESM.eps
Supplementary material 3 Nubp1 and Nubp2 localize at centrioles even after MT depolymerization, indicating that they are integral centriole components in NIH 3T3 cells. A1–A4. Double labeling with anti-α tubulin (red) and anti-Nubp1 (green), and counterstaining for DNA (blue), in untreated cells, serving as control samples. B1–B4. Equivalent panels following depolymerization of MT with nocodazole treatment. Efficient depolymerization is indicated by absence of MTs emanating from centrioles, where Nubp1 labeling is maintained. C1–C4. Control samples with double labeling for α-tubulin (red) and anti-Nubp2 (green), and counterstaining for DNA (blue). D1–D4. Nocodazole-treated equivalent samples, showing Nubp2 labeling in centrioles after MT depolymerization. Scale bars 10 μm. (EPS 10699 kb)
18_2013_1401_MOESM4_ESM.eps
Supplementary material 4 Nubp1 is localized in the axonemes of motile cilia and their basal bodies by immunofluorescence in mouse trachea sections. A1–A2. Hematoxylin/eosin-stained tracheal cross sections from adult mouse at low magnification (A1), showing its characteristic tissue architecture, and detail at higher magnification (A2) revealing the ciliated pseudostratified columnar tracheal epithelium, which was immunolabeled in panels B1–C3. The detail in panel A2 represents the area demarcated with a square and pointed at with an arrow in panel A1. The layer of motile cilia is indicated with black arrows in A2. B1–B3. Immunostaining of tracheal sections, similar to those in A2, using abs against acetylated-tubulin (red, revealing intense labeling of the axonemes in the arrayed motile cilia; B1), Nubp1 (green, revealing a characteristic thin line formed by the consecutive labeled basal bodies as well their corresponding labeled axonemes) and counterstaining for DNA (blue; B2). The overlay image is shown in B3. C1–C3. Equivalent magnified details from images B1–B3, corresponding to the area demarcated in the dotted square in panel B2. The line of labeled basal bodies is indicated by white arrowheads and labeled axonemes by yellow arrowheads. Abbreviations: bronch. bronchiole; bb basal bodies. Scale bars 250 μm (panel A1), 5 μm (panels A2, C1–C3), 10 μm (panels B1–B3). (EPS 18458 kb)
18_2013_1401_MOESM5_ESM.eps
Supplementary material 5 The localization of Nubp1, Nubp2, and KIFC5A to centrioles and basal bodies is not interdependent in NIH 3T3 cells. A1–B3. Double labeling of KIFC5A-silenced cells for γ-tubulin as a centriolar marker (red) and Nubp1 (green) and counterstaining for DNA (blue) in mitotic cells with multi-polar spindles (panels A1–A2) or in interphase cells with amplified centrioles (panels B1–B3) show correct recruitment of Nubp1 in centrioles in the absence of KIFC5A. C1–D3. Equivalent samples as for A1-B3 to show correct Nubp2 localization to centrioles in the absence of KIFC5A. E1–E2. Double labeling of a mitotic Nubp1-silenced cell for γ-tubulin (red) and Nubp1 (green) and counterstaining for DNA (blue) shows normal Nubp2 recruitment to centrioles. F1–F3. Correspondingly, double labeling for acetylated tubulin as an axonemal ciliary marker (red), Nubp1 (green) and DNA (blue) reveals localization of Nubp2 at the basal body in a Nubp1-silenced ciliated cell. All scale bars 10 μm; scale bar in panel F3 is 2.5 μm. (EPS 13506 kb)
18_2013_1401_MOESM6_ESM.eps
Supplementary material 6 Nubp1 is nuclear in interphase, enriched in centrioles throughout the cell cycle, including the mitotic subphases, and localizes to the basal body of the sensory cilium in Xenopus XL177 cells. XL177 cells were labeled with an ab against α-tubulin (red) and an ab against Nubp1 (green). DNA was visualized with Hoechst (blue). (Last row): In serum-deprived XL177 cells, labeled with abs to acetylated tubulin to mark the ciliary axoneme (red), Nubp1 (green), and counterstained for DNA (blue), Nubp1 is detected at the basal body and accompanying daughter centriole. Scale bars 10 μm. (EPS 16450 kb)
18_2013_1401_MOESM7_ESM.eps
Supplementary material 7 A combined silencing and induction of ciliogenesis protocol in NIH 3T3 cells. A. A combined silencing and induction of ciliogenesis protocol allowing the comparative study, in the absence of the depleted protein(s) of interest, of centriole dynamics and ciliogenesis in cycling cells (96 h) and growth-arrested cells (120 h). B1–C2. Control-silenced cells at 96 h (B1, B2) are cycling, as indicated by proliferation marker Ki67 (green) and DNA counterstaining (blue), but are quiescent at 120 h, as evidenced by the lack of Ki-67 signal (C1, C2). Images B1 and C1 were acquired with the same exposure (149.65 ms). D. A random panel of control-silenced cells, by way of an example, labeled for acetylated tubulin (red) and counterstained for DNA (blue) to confirm ciliogenesis, following serum deprivation. In addition to the primary cilium axoneme, acetylated tubulin-containing MTs are visible in some cells, the presence of which is well documented in current literature (reviewed by [102]; [103]). Scale bars 10 μm. (EPS 6638 kb)
18_2013_1401_MOESM8_ESM.eps
Supplementary material 8 Centriole arithmetics and number of nuclei per NIH 3T3 cell at 96 and 120 h in different silencing regimes. A1–C1. Quantification of the number of centrioles per cell in KIFC5A-, Nubp1-, or Nubp2-silenced populations, compared with corresponding control-silenced populations, at 96 h (growth conditions) and after induction of ciliogenesis by serum deprivation at 120 h (cell cycle arrest). A2–C2. Quantification of the number of nuclei per cell in exactly the same silenced populations as above (in corresponding pairs). In panels A1–C2, the averages from four independent experiments are shown and bars represent standard deviation (STD) values. Differences are significant (*), highly significant (**) or extremely significant (***), as indicated, according to two-tailed t test analysis. Abbreviations: Cont.: control silencing; K: KIFC5A-; N1: Nubp1-; N2: Nubp2-silencing. (EPS 581 kb)
18_2013_1401_MOESM9_ESM.eps
Supplementary material 9 Centriole arithmetics and number of nuclei in NIH 3T3 cells at 96 and 120 h in double KIFC5A + Nubp1 silencing. A1–A2. Quantification of the number of centrioles per cell (panel A1) or the number of nuclei per cell (panel A2) in doubly silenced KIFC5A + Nubp1 populations, compared with corresponding control-silenced populations, at 96 h (growth conditions) and after induction of ciliogenesis by serum deprivation at 120 h (cell cycle arrest). Shown are the averages from four independent experiments and bars represent standard deviation (STD) values. Differences are significant (*), highly significant (**) or extremely significant (***), as indicated, according to two-tailed t test analysis. The high incident of multinucleated cells observed for single KIFC5A or Nubp1 silencing was strongly maintained in double-silenced cells at 120 h (13.43 ± 4.55 % vs. 1.15 ± 1.12 %) and was statistically highly and extremely significant in 96 h (p = 0.0068) and 120 h (p = 0.00088) populations (panel A2). The average number of centrioles per cell was comparable in silenced and control populations (panel A1). Abbreviations: Cont.: control silencing; K + N1: KIFC5A + Nubp1 double silencing. (EPS 490 kb)
18_2013_1401_MOESM10_ESM.eps
Supplementary material 10 Protein levels for Nubp1 and Nubp2 are stable throughout the cell cycle in NIH 3T3 cells. A1–C1. Quantification of dimethyl-phospho H3 (panel A1), Nubp1 (panel B1) and Nubp2 protein expression (panel C1) in G1/S synchronized cultures. Values represent average normalized values (ratio of test protein expression to actin expression) from three independent experiments and bars represent standard deviation (STD) values. Differences are significant (*), highly significant (**) or extremely significant (***), as indicated, according to statistical evaluation by one-way ANOVA with Tukey’s test. A2–C3. Corresponding indicative WBs on which this analysis was based. Samples were collected as indicated between 0–14 h, following release from a double thymidine block. The distribution of signal for dimethyl-phospho H3 confirms efficient synchronization and the peak signal at 7 h indicates the timing of mitosis. (EPS 8285 kb)
18_2013_1401_MOESM11_ESM.eps
Supplementary material 11 Nubp1 and Nubp2 protein levels are downregulated upon serum withdrawal in NIH 3T3 cells. A1–B1. Quantification of Nubp1 (panel A1) or Nubp2 (panel B1) protein expression during a time course of serum deprivation in cultured cells. Samples were of cycling cells just prior to serum withdrawal (time point 1), at 12 h after serum withdrawal (point 2), at 24 h after serum withdrawal (point 3) and at 12 h after serum re-introduction (point 4). Values represent average normalized values (ratio of Nubp1 or Nubp2 expression to actin expression) from three independent experiments and bars represent standard deviation (STD) values. Differences were statistically evaluated, as indicated, by one-way ANOVA with Tukey’s test. The statistically significant (*) or highly significant (**) reduction of Nubp1 protein levels during cell cycle exit were down to 62.12 % and the extremely significant (***) reduction of Nubp2 protein levels down to 54.09 % of initial levels in cycling conditions. A2–B2. Corresponding indicative Western blots on which this analysis was based. (EPS 3106 kb)
18_2013_1401_MOESM12_ESM.eps
Supplementary material 12 Mother centriole-specific protein cenexin is localized in amplified centrioles of silenced NIH 3T3 cells and at the basal body of the cilium under the different silencing regimes. A–C. Silencing at 96 h (cycling cells). Double staining for γ-tubulin as a marker for centrioles (red) and for cenexin (green) and counterstaining for DNA (blue) in control-silenced cells (A), Nubp1- (B), and KIFC5A-silenced cells (C). In control-silenced cells, cenexin is detected in only one of the two centrioles: in the images the mother centriole is indicated with a yellow arrowhead and the daughter centriole with a red arrowhead. In silenced cells, most or all amplified centrioles are cenexin-positive. D–F. Silencing at 120 h (serum-deprived cells). Panels with double staining for acetylated-tubulin as a marker for the ciliary axoneme (red) and for cenexin (green) and counterstaining for DNA (blue) in control-silenced cells (D), Nubp1- (E), and Nubp2-silenced cells (F). Scale bars 10 μm; details in the accompanying small images are shown at double the magnification of their corresponding main image. (EPS 9651 kb)
18_2013_1401_MOESM13_ESM.eps
Supplementary material 13 CP110 and CEP290 are normally recruited to the centrosome and basal body under the different silencing regimes in NIH 3T3 cells. A1–A4. Silencing at 96 h (cycling cells). Double staining for γ-tubulin as a marker for centrioles (red) and for CP110 (green) and counterstaining for DNA (blue) in control-silenced cells (A1), Nubp1- (A2), Nubp2- (A3), and KIFC5A-silenced cells (A4). B1–B4. Silencing at 120 h (serum-deprived cells). Equivalent panels with double staining of silenced cells for acetylated-tubulin as a marker for the ciliary axoneme (red) and for CP110 (green) and counterstaining for DNA (blue). C1–C3. Silencing at 96 h (cycling cells). Double staining for γ-tubulin (red) and for CEP290 (green) and counterstaining for DNA (blue) in control-silenced cells (C1), Nubp1- (C2), and KIFC5A-silenced cells (C3). D1–D3. Silencing at 120 h (serum-deprived cells). Equivalent panels with double staining of silenced cells for acetylated-tubulin (red) and for CEP290 (green) and counterstaining for DNA (blue). Scale bars 10 μm; details in the accompanying small images are shown at double the magnification of their corresponding main image. (EPS 22850 kb)
18_2013_1401_MOESM14_ESM.eps
Supplementary material 14 Localization of Aurora A kinase (AurA) and the GTPase Rab8 in centrioles and basal body of the cilium under the different silencing regimes in NIH 3T3 cells. A1–C3. Silencing at 96 h (cycling cells). Double staining for α-tubulin as a marker for MTs in normal and multi-polar spindles (red) and for AurA (green) and counterstaining for DNA (blue) in control-silenced cells (A1–A3), Nubp1- (B1–B3), and KIFC5A-silenced cells (C1-C3). D–H. Silencing at 120 h (serum-deprived cells). Panels with double staining for acetylated-tubulin as a marker for the ciliary axoneme (red) and for Rab8 (green) in control-silenced cells (D), Nubp1- (E), Nubp2- (F), KIFC5A- (G) and double KIFC5A + Nubp1-silenced cells (H). Scale bars 10 μm; details in the accompanying small images are shown at double the magnification of their corresponding main image. (EPS 11860 kb)
18_2013_1401_MOESM15_ESM.eps
Supplementary material 15 Chaperones CCT8 and CCT4, and BBSome protein BBS7 are normally recruited to the basal body under the different silencing regimes in NIH 3T3 cells. A–E. Silencing at 120 h (serum-deprived cells). Panels with double staining for acetylated-tubulin as a marker for the ciliary axoneme (red), for CCT8 (green) and counterstaining for DNA (blue) in control-silenced cells (A), Nubp1- (B), Nubp2- (C), KIFC5A- (D) and double KIFC5A + Nubp1-silenced cells (E). F–G. Labeling for acetylated-tubulin (red), CCT4 (green) and counterstaining for DNA (blue) in control- (F), and Nubp1- silenced cells (G) at 120 h. H–K. Different silencing regimes, as indicated, at 120 h. Labeling for acetylated-tubulin (red), and BBS7 (green). Scale bars 10 μm; details in the accompanying small images are shown at double the magnification of their corresponding main image. (EPS 26062 kb)
18_2013_1401_MOESM17_ESM.doc
Supplementary material 17 Identification of proteins contained in Nubp1 immunoprecipitates (bands 1-7 in Fig. 8) by LC–MS/MS. (DOC 63 kb)
18_2013_1401_MOESM18_ESM.docx
Supplementary material 18 Summary of effects of different silencing regimes on ciliogenesis and centriole arithmetics, as presented in this work or by Christodoulou et al. 2006 [49]. The descriptions used in each case refer to statistically significant changes only (otherwise the term “unchanged” is used). (DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Kypri, E., Christodoulou, A., Maimaris, G. et al. The nucleotide-binding proteins Nubp1 and Nubp2 are negative regulators of ciliogenesis. Cell. Mol. Life Sci. 71, 517–538 (2014). https://doi.org/10.1007/s00018-013-1401-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1401-6