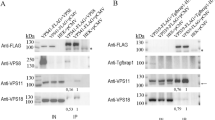
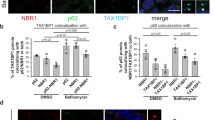
Abstract
Multisubunit protein complexes are assembled in the endoplasmic reticulum (ER). Existing pools of single subunits and assembly intermediates ensure the efficient and rapid formation of complete complexes. While being kinetically beneficial, surplus components must be eliminated to prevent potentially harmful accumulation in the ER. Surplus single chains are cleared by the ubiquitin–proteasome system. However, the fate of not secreted assembly intermediates of multisubunit proteins remains elusive. Here we show by high-resolution double-label confocal immunofluorescence and immunogold electron microscopy that naturally occurring surplus fibrinogen Aα–γ assembly intermediates in HepG2 cells are dislocated together with EDEM1 from the ER to the cytoplasm in ER-derived vesicles not corresponding to COPII-coated vesicles originating from the transitional ER. This route corresponds to the novel ER exit path we have previously identified for EDEM1 (Zuber et al. Proc Natl Acad Sci USA 104:4407–4412, 2007). In the cytoplasm, detergent-insoluble aggregates of fibrinogen Aα–γ dimers develop that are targeted by the selective autophagy cargo receptors p62/SQSTM1 and NBR1. These aggregates are degraded by selective autophagy as directly demonstrated by high-resolution microscopy as well as biochemical analysis and inhibition of autophagy by siRNA and kinase inhibitors. Our findings demonstrate that different pathways exist in parallel for ER-to-cytoplasm dislocation and subsequent proteolytic degradation of large luminal protein complexes and of surplus luminal single-chain proteins. This implies that ER-associated protein degradation (ERAD) has a broader function in ER proteostasis and is not limited to the elimination of misfolded glycoproteins.








Similar content being viewed by others
References
Zuber C, Cormier JH, Guhl B, Santimaria R, Hebert DN, Roth J (2007) EDEM1 reveals a quality control vesicular transport pathway out of the endoplasmic reticulum not involving the COPII exit sites. Proc Natl Acad Sci USA 104:4407–4412
Hiller MM, Finger A, Schweiger M, Wolf DH (1996) ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273:1725–1728
Braakman I, Bulleid NJ (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 80:71–99
Bonifacino JS, Weissman AM (1998) Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol 14:19–57
Christis C, Lubsen N, Braakman I (2008) Protein folding includes oligomerization: examples from the endoplasmic reticulum and cytosol. FEBS J 275:4700–4727
Bagola K, Mehnert M, Jarosch E, Sommer T (2011) Protein dislocation from the ER. Biochim Biophys Acta 1808:925–936
Weisel JW (2005) Fibrinogen and fibrin. Adv Protein Chem 70:247–299
Redman CM, Xia H (2001) Fibrinogen biosynthesis. Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann NY Acad Sci 936:480–495
Mosesson MW, Siebenlist KR, Meh DA (2001) The structure and biological features of fibrinogen and fibrin. Ann NY Acad Sci 936:11–30
Yu S, Sher B, Kudryk B, Redman CM (1984) Fibrinogen precursors. Order of assembly of fibrinogen chains. J Biol Chem 259:10574–10581
Huang S, Cao Z, Chung DW, Davie EW (1996) The role of beta-gamma and alpha-gamma complexes in the assembly of human fibrinogen. J Biol Chem 271:27942–27947
Blomback B, Hessel B, Hogg D (1976) Disulfide bridges in NH2-terminal part of human fibrinogen. Thrombosis Res 8:639–658
Huang S, Cao Z, Davie EW (1993) The role of amino-terminal disulfide bonds in the structure and assembly of human fibrinogen. Biochem Biophys Res Commun 190:488–495
Henschen A (1978) Disulfide bridges in the middle part of human fibrinogen. Hoppe-Seyler’s Z Physiol Chemie 359:1757–1770
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Yang M, Omura S, Bonifacino JS, Weissman AM (1998) Novel aspects of degradation of T cell receptor subunits from the endoplasmic reticulum (ER) in T cells: importance of oligosaccharide processing, ubiquitination, and proteasome-dependent removal from ER membranes. J Exp Med 187:835–846
Huppa JB, Ploegh HL (1997) The alpha chain of the T cell antigen receptor is degraded in the cytosol. Immunity 7:113–122
Yu H, Kaung G, Kobayashi S, Kopito RR (1997) Cytosolic degradation of T-cell receptor alpha chains by the proteasome. J Biol Chem 272:20800–20804
Bonifacino JS, Suzuki CK, Lippincott-Schwartz J, Weissman AM, Klausner RD (1989) Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol 109:73–83
Tiwari S, Weissman AM (2001) Endoplasmic reticulum (ER)-associated degradation of T cell receptor subunits. Involvement of ER-associated ubiquitin-conjugating enzymes (E2 s). J Biol Chem 276:16193–16200
Bonifacino J, McCarthy S, Maguire J, Nakayama T, Singer D, Klausner R, Singer A (1990) Novel post-translational regulation of TCR expression in CD4+ CD8+ thymocytes influenced by CD4. Nature 344:247–251
Kearse K, Roberts J, Munitz T, Wiest D, Nakayama T, Singer A (1994) Developmental regulation of αβ T cell antigen receptor expression results from differential stability of nascent TCR-α proteins within the endoplasmic reticulum of immature and mature T cells. EMBO J 13:4504–4514
Carvalho P, Goder V, Rapoport TA (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126:361–373
Sato BK, Schulz D, Do PH, Hampton RY (2009) Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol Cell 34:212–222
Roy S, Yu S, Banerjee D, Overton O, Mukhopadhyay G, Oddoux C, Grieninger G, Redman C (1992) Assembly and secretion of fibrinogen. Degradation of individual chains. J Biol Chem 267:23151–23158
Xia H, Redman C (1999) The degradation of nascent fibrinogen chains is mediated by the ubiquitin proteasome pathway. Biochem Biophys Res Commun 261:590–597
Carvalho P, Stanley AM, Rapoport TA (2010) Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell 143:579–591
Denic V, Quan EM, Weissman JS (2006) A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126:349–359
Molinari M, Calanca V, Galli C, Lucca P, Paganetti P (2003) Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science 299:1397–1400
Oda Y, Hosokawa N, Wada I, Nagata K (2003) EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 299:1394–1397
Cormier JH, Tamura T, Sunryd JC, Hebert DN (2009) EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Mol Cell 34:627–633
Gauss R, Kanehara K, Carvalho P, Ng DTW, Aebi M (2011) A complex of Pdi1p and the mannosidase Htm1p initiates clearance of unfolded glycoproteins from the endoplasmic reticulum. Mol Cell 42:782–793
Quan EM, Kamiya Y, Kamiya D, Denic V, Weibezahn J, Kato K, Weissman JS (2008) Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol Cell 32:870–877
Olivari S, Cali T, Salo KE, Paganetti P, Ruddock LW, Molinari M (2006) EDEM1 regulates ER-associated degradation by accelerating de-mannosylation of folding-defective polypeptides and by inhibiting their covalent aggregation. Biochem Biophys Res Commun 349:1278–1284
Hosokawa N, Tremblay LO, Sleno B, Kamiya Y, Wada I, Nagata K, Kato K, Herscovics A (2010) EDEM1 accelerates the trimming of α1,2-linked mannose on the C branch of N-glycans. Glycobiology 20:567–575
Clerc S, Hirsch C, Oggier DM, Deprez P, Jakob C, Sommer T, Aebi M (2009) Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol 184:159–172
Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R (2012) COPII and the regulation of protein sorting in mammals. Nat Cell Biol 14(1):20–28
Bannykh SI, Rowe T, Balch WE (1996) The organization of endoplasmic reticulum export complexes. J Cell Biol 135:19–35
Strous GJ, Van Kerkhof P, Brok R, Roth J, Brada D (1987) Glucosidase II, a protein of the endoplasmic reticulum with high mannose oligosaccharide chains and a rapid turnover. J Biol Chem 262:3620–3625
Wu Y, Termine DJ, Swulius MT, Moremen KW, Sifers RN (2007) Human endoplasmic reticulum mannosidase I is subject to regulated proteolysis. J Biol Chem 282:4841–4849
Le Fourn V, Gaplovska-Kysela K, Guhl B, Santimaria R, Zuber C, Roth J (2009) Basal autophagy is involved in the degradation of the ERAD component EDEM1. Cell Mol Life Sci 66:1434–1445
Cali T, Galli C, Olivari S, Molinari M (2008) Segregation and rapid turnover of EDEM1 by an autophagy-like mechanism modulates standard ERAD and folding activities. Biochem Biophys Res Commun 371:405–410
Roth J, Bendayan M, Orci L (1978) Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem 26:1074–1081
Roth J (1982) The protein A-gold (pAg) technique—a qualitative and quantitative approach for antigen localization on thin sections. In: Bullock G, Petrusz P (eds) Techniques in immunocytochemistry, vol 1. Academic Press, London, pp 108–133
Tokuyasu K (1989) Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J 21:163–171
Tokuyasu K (1978) A study of positive staining of ultrathin frozen sections. J Ultrastruct Res 63:287–307
Roth J, Taatjes DJ, Warhol MJ (1989) Prevention of non-specific interactions of gold-labeled reagents on tissue sections. Histochemistry 92:47–56
Hartwig R, Danishefsky KJ (1991) Studies on the assembly and secretion of fibrinogen. J Biol Chem 266:6578–6585
Huang S, Mulvihill ER, Farrell DH, Chung DW, Davie EW (1993) Biosynthesis of human fibrinogen. Subunit interactions and potential intermediates in the assembly. J Biol Chem 268:8919–8926
Roy S, Sun A, Redman C (1996) In vitro assembly of the component chains of fibrinogen requires endoplasmic reticulum factors. J Biol Chem 271:24544–24550
Vashist S, Ng DT (2004) Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J Cell Biol 165:41–52
Palade G (1975) Intracellular aspects of the process of protein biosynthesis. Science 189:347–358
Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R (2011) COPII and the regulation of protein sorting in mammals. Nat Cell Biol 14:20–28
Bannykh SI, Balch WE (1997) Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol 138:1–4
Yorimitsu T, Klionsky DJ (2005) Autophagy: molecular machinery for self-eating. Cell Death Differ 12(Suppl 2):1542–1552
Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132
Eskelinen E-L, Reggiori F, Baba M, Kovacs AL, Seglen PO (2011) Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy 7:935–956
Pavelka M, Roth J (2010) Functional ultrastructure. An atlas of tissue biology and pathology, 2nd edn. Springer, Vienna
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P (2000) Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275:992–998
Taguchi-Atarashi N, Hamasaki M, Matsunaga K, Omori H, Ktistakis NT, Yoshimori T, Noda T (2010) Modulation of local PtdIns3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy. Traffic 11:468–478
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM (2010) Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285:10850–10861
Kochl R, Hu XW, Chan EY, Tooze SA (2006) Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 7:129–145
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Kirkin V, Lamark T, Johansen T, Dikic I (2009) NBR1 cooperates with p62 in selective autophagy of ubiquitinated targets. Autophagy 5:732–733
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115:727–738
Furumai R, Komatsu Y, Nishino N, Khochbin S, Yoshida M, Horinouchi S (2001) Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. Proc Natl Acad Sci USA 98:87–92
Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, Bilusic I, Theurillat JP, Overvatn A, Ishii T, Elazar Z, Komatsu M, Dikic I, Johansen T (2009) A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 33:505–516
Bordallo J, Plemper RK, Finger A, Wolf DH (1998) De3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell 9:209–222
Werner ED, Brodsky JL, McCracken AA (1996) Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci USA 93:13797–13801
Teckman JH, Perlmutter DH (1996) The endoplasmic reticulum degradation pathway for mutant secretory proteins alpha 1-antitrypsin Z and S is distinct from that for an unassembled membrane protein. J Biol Chem 271:13215–13220
Osborne AR, Rapoport TA, van den Berg B (2005) Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol 21:529–550
Bonardi F, Halza E, Walko M, Du Plessis Fß, Nouwen N, Feringa BL, Driessen AJM (2011) Probing the SecYEG translocation pore size with preproteins conjugated with sizable rigid spherical molecules. Proc Natl Acad Sci USA 108:7775–7780
Tian P, Andricioaei I (2006) Size, motion, and function of the SecY translocon revealed by molecular dynamics simulations with virtual probes. Biophys J 90:2718–2730
Yermolenko IS, Lishko VK, Ugarova TP, Magonov SN (2011) High-resolution visualization of fibrinogen molecules and fibrin fibers with atomic force microscopy. Biomacromolecules 12:370–379
Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF (2009) Crystal structure of human fibrinogen. Biochemistry 48:3877–3886
Mosesson MW, Hainfeld J, Wall J, Haschemeyer RH (1981) Identification and mass analysis of human fibrinogen molecules and their domains by scanning transmission electron microscopy. J Mol Biol 153:695–718
Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, Katada T (2011) cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell 22:2301–2308
Malhotra V, Erlmann P (2011) Protein export at the ER: loading big collagens into COPII carriers. EMBO J 30:3475–3480
Palade GE (1956) Intracisternal granules in the exocrine cells of the pancreas. J Biophys Biochem Cytol 2:417–422
Geuze HJ, Slot JW (1980) Disproportional immunostaining patterns of two secretory proteins in guinea pig and rat exocrine pancreatic cells. An immunoferritin and fluorescence study. Eur J Cell Biol 21:93–100
Kim PS, Bole D, Arvan P (1992) Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol 118:541–549
Alanen A, Pira U, Lassila O, Roth J, Franklin RM (1985) Mott cells are plasma cells defective in immunoglobulin secretion. Eur J Immunol 15:235–242
Brewer JW, Randall TD, Parkhouse RM, Corley RB (1994) Mechanism and subcellular localization of secretory IgM polymer assembly. J Biol Chem 269:17338–17348
Lomas DA, Evans DL, Finch JT, Carrell RW (1992) The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature 357:605–607
Lin L, Schmidt B, Teckman J, Perlmutter DH (2001) A naturally occurring nonpolymerogenic mutant of alpha 1-antitrypsin characterized by prolonged retention in the endoplasmic reticulum. J Biol Chem 276:33893–33898
Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, Yoshimori T (2006) Intracellular inclusions containing mutant alpha 1-antitrypsin Z are propagated in the absence of autophagic activity. J Biol Chem 281:4467–4476
Yam GHF, Gaplovska-Kysela K, Zuber C, Roth J (2007) Aggregated myocilin induces Russell bodies and causes apoptosis - Implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol 170:100–109
Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, Helenius A (2007) Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131:516–529
Lilley BN, Gilbert JM, Ploegh HL, Benjamin TL (2006) Murine polyomavirus requires the endoplasmic reticulum protein Derlin-2 to initiate infection. J Virol 80:8739–8744
Acknowledgments
We thank W.S. Hancock (Boston, MA), N.-O. Ku (Seoul), P.M. Lackie (Southampton), Jon Sonderholm (Seoul), D.J. Taatjes (Burlington, VT) and G.H.F. Yam (Hong Kong) for critical reading of the manuscript. We are grateful to Tamara Locher and Roger Santimaria for skillful technical assistance. Insook Jang is recipient of a fellowship from the Brain Korea 21 program. Funding was received by the Swiss National Science Foundation (to J.R.), the Canton of Zurich (to J.R.), by the World Class University Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10086-0) (to J.R. and J.W.C), the National Research Foundation of Korea by the Ministry of Education, Science and Technology (2010-0027736) (to J.R. and J.W.C) and partly by the National Research Foundation funded by the Korean Government (2012R1A2A1A05026333) (to J.W.C.).
Conflict of interest
The authors declare they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Le Fourn, S. Park, and I. Jang equally contributed to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Le Fourn, V., Park, S., Jang, I. et al. Large protein complexes retained in the ER are dislocated by non-COPII vesicles and degraded by selective autophagy. Cell. Mol. Life Sci. 70, 1985–2002 (2013). https://doi.org/10.1007/s00018-012-1236-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1236-6