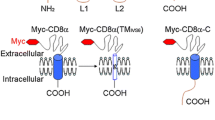
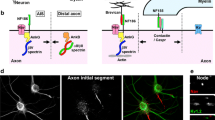
Abstract
Neuronal action potentials are generated through voltage-gated sodium channels, which are tethered by ankyrinG at the membrane of the axon initial segment (AIS). Despite the importance of the AIS in the control of neuronal excitability, the cellular and molecular mechanisms regulating sodium channel expression at the AIS remain elusive. Our results show that GSK3α/β and β-catenin phosphorylated by GSK3 (S33/37/T41) are localized at the AIS and are new components of this essential neuronal domain. Pharmacological inhibition of GSK3 or β-catenin knockdown with shRNAs decreased the levels of phosphorylated-β-catenin, ankyrinG, and voltage-gated sodium channels at the AIS, both “in vitro” and “in vivo”, therefore diminishing neuronal excitability as evaluated via sodium current amplitude and action potential number. Thus, our results suggest a mechanism for the modulation of neuronal excitability through the control of sodium channel density by GSK3 and β-catenin at the AIS.








Similar content being viewed by others
References
Kole MH et al (2008) Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11:178–186
Kole MH, Letzkus JJ, Stuart GJ (2007) Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55:633–647
Ango F et al (2004) Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119:257–272
Garrido JJ et al (2003) A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300:2091–2094
Ogawa Y et al (2008) Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci 28:5731–5739
Zhou D et al (1998) AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143:1295–1304
Nakada C et al (2003) Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol 5:626–632
Palay SL, Sotelo C, Peters A, Orkand PM (1968) The axon hillock and the initial segment. J Cell Biol 38:193–201
Sanchez-Ponce D, Munoz A, Garrido JJ (2011) Casein kinase 2 and microtubules control axon initial segment formation. Mol Cell Neurosci 46:222–234
Tapia M, Wandosell F, Garrido JJ (2010) Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS One 5:e12908
Winckler B, Forscher P, Mellman I (1999) A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397:698–701
Konishi Y, Setou M (2009) Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci 12:559–567
Song AH et al (2009) A selective filter for cytoplasmic transport at the axon initial segment. Cell 136:1148–1160
Hedstrom KL, Ogawa Y, Rasband MN (2008) AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183:635–640
Sobotzik JM et al (2009) AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci USA 106:17564–17569
Ciani L, Salinas PC (2007) c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability. BMC Cell Biol 8:27
Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29:95–102
Morfini G et al (2004) A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J 23:2235–2245
Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42:897–912
Tyagarajan SK et al (2011) Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci USA 108:379–384
Gottardi CJ, Gumbiner BM (2004) Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167:339–349
Liu C et al (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847
Hart M et al (1999) The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol 9:207–210
Faux MC, Coates JL, Kershaw NJ, Layton MJ, Burgess AW (2010) Independent interactions of phosphorylated beta-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions. PLoS One 5:e14127
Maher MT, Mo R, Flozak AS, Peled ON, Gottardi CJ (2010) Beta-catenin phosphorylated at serine 45 is spatially uncoupled from beta-catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS One 5:e10184
Huang P, Senga T, Hamaguchi M (2007) A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene 26:4357–4371
Shaw RM et al (2007) Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128:547–560
Ligon LA, Karki S, Tokito M, Holzbaur EL (2001) Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol 3:913–917
Yu X, Malenka RC (2003) Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci 6:1169–1177
Yu X, Malenka RC (2004) Multiple functions for the cadherin/catenin complex during neuronal development. Neuropharmacology 47:779–786
Haegel H et al (1995) Lack of beta-catenin affects mouse development at gastrulation. Development 121:3529–3537
Campos VE, Du M, Li Y (2004) Increased seizure susceptibility and cortical malformation in beta-catenin mutant mice. Biochem Biophys Res Commun 320:606–614
Votin V, Nelson WJ, Barth AI (2005) Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J Cell Sci 118:5699–5708
Lesage F, Hibino H, Hudspeth AJ (2004) Association of beta-catenin with the alpha-subunit of neuronal large-conductance Ca2+-activated K+ channels. Proc Natl Acad Sci USA 101:671–675
Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415
Debanne D et al (2008) Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat Protoc 3:1559–1568
Chilov D et al (2011) Phosphorylated beta-catenin localizes to centrosomes of neuronal progenitors and is required for cell polarity and neurogenesis in developing midbrain. Dev Biol 357:259–268
Sanchez-Ponce D, Tapia M, Munoz A, Garrido JJ (2008) New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci 37:832–844
Pan Z et al (2006) A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 26:2599–2613
Rasband MN (2010) The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11:552–562
Winckler B, Mellman I (1999) Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron 23:637–640
Leterrier C et al (2011) End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc Natl Acad Sci USA 108(21):8826–8831
Nakata T, Hirokawa N (2003) Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol 162:1045–1055
Kim WY et al (2006) Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52:981–996
Bhat R et al (2003) Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem 278:45937–45945
Hedstrom KL et al (2007) Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol 178:875–886
Jenkins SM, Bennett V (2001) Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155:739–746
Nadri C, Dean B, Scarr E, Agam G (2004) GSK-3 parameters in postmortem frontal cortex and hippocampus of schizophrenic patients. Schizophr Res 71:377–382
Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA (2009) Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacology 34:2112–2124
Brachet A et al (2010) Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. J Cell Biol 191:383–395
Peineau S et al (2009) A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol Brain 2:22
Peineau S et al (2007) LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53:703–717
Zhu LQ et al (2007) Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci 27:12211–12220
Peng YR et al (2009) Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron 61:71–84
Zonta B et al (2011) A critical role for neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron 69:945–956
Kapfhamer D et al (2010) Protein phosphatase 2a and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-Type potassium channel activity. J Neurosci 30:8830–8840
Daugherty RL, Gottardi CJ (2007) Phospho-regulation of beta-catenin adhesion and signaling functions. Physiology (Bethesda) 22:303–309
Mo R et al (2009) The terminal region of beta-catenin promotes stability by shielding the Armadillo repeats from the axin-scaffold destruction complex. J Biol Chem 284:28222–28231
Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M (1999) Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem 274:36734-36740
David MD et al (2008) Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential beta-catenin phosphorylation. J Cell Sci 121:2718–2730
Staal FJ, Noort Mv M, Strous GJ, Clevers HC (2002) Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep 3:63-68
Hendriksen J et al (2008) Plasma membrane recruitment of dephosphorylated {beta}-catenin upon activation of the Wnt pathway. J Cell Sci 121:1793–1802
Winston JT et al (1999) The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev 13:270–283
Su Y et al (2008) APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell 32:652–661
Song DH et al (2003) CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem 278:24018–24025
Brechet A et al (2008) Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol 183:1101–1114
Lovestone S, Killick R, Di Forti M, Murray R (2007) Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci 30:142–149
Acknowledgments
The authors would like to acknowledge Hector Diez, Sylvain Rama, Alfonso Araque, and Michael Seagar for their advice and critical reading of the manuscript. This work was supported by grants SAF2009-12249-C02-02 and SAF2009-12249-C02-01 from the Ministerio de Ciencia e Innovacion (Spain), by the Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED, Spain), INSERM and Agence National de la Recherche (EXCION, EPISOM). Mónica Tapia is supported by a CSIC-JAE fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2012_1059_MOESM1_ESM.pdf
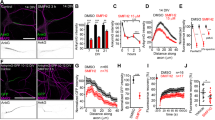
Supplementary material 1 Fig. S1. β-catenin interference shRNAs suppress β-catenin-pS33/37/T41 expression at the AIS (A) Endogenous β-catenin and β-catenin-pS33/37/T41 in N2a cells is suppressed by the expression of two different β-catenin shRNAs. β-catenin shRNA1 reduced endogenous β-catenin and β-catenin-pS33/37/T41 expression approximately 70 %, while β-catenin shRNA2 reduced them only by 50 %. Graph represents the mean and SD of β-catenin expression levels normalized to β-actin levels in 3 different experiments. ***p < 0.001, **p < 0.01, t test. (B,C) Representative images of hippocampal neurons nucleofected with β-catenin shRNAs or scrambled shRNA plasmids. Neurons were fixed after 3 days and stained with β-catenin (B) or pS33/37T41-β-catenin (C) antibodies as indicated. (D) 6-DIV hippocampal neurons nucleofected with β-catenin shRNA 1 or scrambled shRNA plasmids. Neurons were stained with anti-MAP2, and β-catenin-pS33/37/T41 or β-catenin antibodies. Nucleofected neurons were identified by their RFP fluorescence. High magnification at the level of AIS (boxes) of non-nucleofected, β-catenin shRNA 1 or scrambled shRNA nucleofected neurons is shown. White arrows indicate the soma of non-transfected neurons and red arrows indicate transfected neurons. Arrowheads indicate the position of axons in neurons stained with β-catenin and AIS in neurons stained with pS33/37T41-β-catenin. Scale bar = 50 µm.Fig. S2. β-catenin-pS33/37/T41 staining is also localized at the centrosome in early development neurons.24-h neurons were fixed and stained with β-catenin-pS33/37/T41 (green) and γ-tubulin (red) antibodies. The arrows indicate centrosome position. Scale bar = 25 μm.Fig. S3. Quantification of β-catenin-pS33/37/T41 fluorescence intensity along neurons at different developmental stagesImages of different developmental stages hippocampal neurons stained with anti-β-catenin-pS33/37/T41 antibody (green), as shown in Fig. 1. Graphs represent the β-catenin-pS33/37/T41 fluorescence intensity along a line designed through dendrites-soma and axon. Fluorescence intensity was quantified in gray scale images. Scale bar = 50 μm.Fig. S4. β-catenin-pS45 is located at the nucleus but not at the AIS in hippocampal neurons.Hippocampal neurons from different stages were stained with an antibody that recognizes β-catenin when is phosphorylated at serine 45 and anti-tyrosinated-α-tubulin (top panels, 24-h neurons) or anti-PanNaCh (bottom panels). Note that β-catenin-pS45 is concentrated in nucleus at both stages, but not at the AIS.Fig. S5. Proteasome inhibition does not alter pS33/37T41-β-catenin expression at the AIS, but increases its expression at neuronal soma.(A) β-catenin and β-catenin-pS33/37/T41 expression in cortical neurons treated or not with MG132 50 nM for 24 h. Graph represents the mean ± SEM protein expression levels normalized to β-actin levels in three different experiments. **p < 0.01, *p < 0.05, t test. (B) Hippocampal neurons treated with proteasome inhibitor MG132 from 5 DIV to 6 DIV and stained with anti-β-catenin-pS33/37/T41 and anti-MAP2 antibodies. Note the increased fluorescence intensity of β-catenin-pS33/37/T41 at the neuronal soma. Arrows indicate AIS position and arrowheads soma position. All images were acquired in same intensity fluorescence conditions. Scale bar = 50 µmFig. S6. β-catenin related proteins, APC and N-cadherin, are not localized at the axon initial segment.Hippocampal neurons cultured for 2 DIV (A) and 6 DIV (B and C) were stained with pS33/37T41-β-catenin antibody (green), and APC (B) or N-cadherin (C) antibodies (red). Note that pS33/37T41-β-catenin colocalizes with APC along axonal microtubules and growth cone at 2 DIV, while neither APC nor N-cadherin expression is detected at the AIS of 6-DIV hippocampal neurons. Insets show enlarged views of axon initial segments. Scale bar = 50µm.Fig. S7. Percentage of β-catenin shRNAs nucleofected neurons at different developmental stages, and with different β-catenin-pS33/37/T41, ankyrinG and sodium channels intensities at the AIS.Summarized results of three categories (high expression, weak expression, and absence of expression) of neurons established in function of the staining intensity compared to control neurons mean staining. Results are the mean ± SEM percentage of neurons in each category from three independent experiments (100 neurons/experimental condition and experiment). ***p < 0.001 and **p < 0.01 compared to scrambled shRNA, t test. Neurons showing total fluorescence intensity over 50 % of control neurons (scrambled shRNA) were considered as high intensity category, and neurons with total fluorescence intensity equal or fewer than 50 % of control neurons were considered in the weak intensity category. Sc = scramble shRNA, Sh1 = β-catenin shRNA 1, Sh2 = β-catenin shRNA 2.Fig. S8. β-catenin suppression slows down axonal elongation and dendritic maturation.(A) 3-DIV neurons stained for MAP2 and tau-1 to identify the axon and somatodendritic compartment. The graph represents the axonal length of neurons nucleofected with scrambled and β-catenin shRNAs. Both shRNAs did not impair axon formation, but slightly reduced the length of the axon, identified by the axonal marker Tau-1 (165.58 ± 7.55 µm or 186.28 ± 7.09 µm compared to 227.75 ± 8.92 µm in scrambled shRNA nucleofected neurons). Data represent the mean ± SEM of three independent experiments (50 neurons/experimental condition and experiment). (B) 6-DIV neurons stained with MAP2 and βIII-tubulin antibodies to show dendritic development of β-catenin shRNA 1, β-catenin shRNA 2, or scrambled shRNA-nucleofected neurons. Nucleofected neurons were identified by their RFP fluorescence. Graph represents the mean ± SEM of mean dendritic length from three independent experiments (25 neurons/experimental condition in each experiment). Scale bar = 50 µm. ***p < 0.001, **p < 0.01, paired t test.(PDF 2387 kb)
Rights and permissions
About this article
Cite this article
Tapia, M., Del Puerto, A., Puime, A. et al. GSK3 and β-catenin determines functional expression of sodium channels at the axon initial segment. Cell. Mol. Life Sci. 70, 105–120 (2013). https://doi.org/10.1007/s00018-012-1059-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1059-5