Abstract
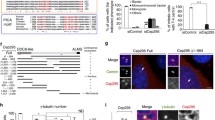
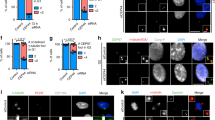
Dictyostelium centrosomes consist of a layered core structure surrounded by a microtubule-nucleating corona. At the G2/M transition, the corona dissociates and the core structure duplicates, yielding two spindle pole bodies. Finally, in telophase, the spindle poles mature into two new, complete centrosomes. CP55 was identified in a centrosomal proteome analysis. It is a component of the centrosomal core structure, and persists at the centrosome throughout the entire cell cycle. FRAP experiments revealed that during interphase the majority of centrosomal GFP-CP55 is immobile, which indicates a structural task of CP55 at the centrosome. The CP55null mutant is characterized by increased ploidy, a less structured, slightly enlarged corona, and by supernumerary, cytosolic MTOCs, containing only corona proteins and lacking a core structure. Live cell imaging showed that supernumerary MTOCs arise in telophase. Lack of CP55 also caused premature recruitment of the corona organizer CP148 to mitotic spindle poles, already in metaphase instead of telophase. Forces transmitted through astral microtubules may expel prematurely acquired or loosely attached corona fragments into the cytosol, where they act as independent MTOCs. CP55null cells were also impaired in growth, most probably due to difficulties in centrosome splitting during prophase. Furthermore, although they were still capable of phagocytosis, they appeared unable to utilize phagocytosed nutrients. This inability may be attributed to their partially disorganized Golgi apparatus.









Similar content being viewed by others
References
Azimzadeh J, Marshall WF (2010) Building the centriole. Curr Biol 20:R816–R825
Bettencourt-Dias M, Glover DM (2009) SnapShot: centriole biogenesis. Cell 136(188–188):e181
Hatch E, Stearns T (2011) The life cycle of centrioles. Cold Spring Harb Symp Quant Biol 75:425–431
Haren L, Stearns T, Lüders J (2009) Plk1-dependent recruitment of γ-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS ONE 4:e5976
Dictenberg JB, Zimmerman W, Sparks CA, Young A, Vidair C, Zheng YX, Carrington W, Fay FS, Doxsey SJ (1998) Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol 141:163–174
Fong KW, Choi YK, Rattner JB, Qi RZ (2008) CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the γ-tubulin ring complex. Mol Biol Cell 19:115–125
Gräf R, Daunderer C, Schulz I (2004) Molecular and functional analysis of the dictyostelium centrosome. Int Rev Cytol 241:155–202
Euteneuer U, Gräf R, Kube-Granderath E, Schliwa M (1998) Dictyostelium γ-tubulin: molecular characterization and ultrastructural localization. J Cell Sci 111:405–412
Daunderer C, Gräf R (2002) Molecular analysis of the cytosolic Dictyostelium γ-tubulin complex. Eur J Cell Biol 81:175–184
Daunderer C, Schliwa M, Gräf R (2001) Dictyostelium centrin-related protein (DdCrp), the most divergent member of the centrin family, possesses only two EF hands and dissociates from the centrosome during mitosis. Eur J Cell Biol 80:621–630
Rehberg M, Gräf R (2002) Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol Biol Cell 13:2301–2310
Rehberg M, Kleylein-Sohn J, Faix J, Ho TH, Schulz I, Gräf R (2005) Dictyostelium LIS1 is a centrosomal protein required for microtubule/cell cortex interactions, nucleus/centrosome linkage, and actin dynamics. Mol Biol Cell 16:2759–2771
Gräf R, Euteneuer U, Ho TH, Rehberg M (2003) Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol Biol Cell 14:4067–4074
Schulz I, Erle A, Gräf R, Krüger A, Lohmeier H, Putzler S, Samereier M, Weidenthaler S (2009) Identification and cell cycle-dependent localization of nine novel, genuine centrosomal components in Dictyostelium discoideum. Cell Motil Cytoskelet 66:915–928
Blau-Wasser R, Euteneuer U, Xiong H, Gassen B, Schleicher M, Noegel AA (2009) CP250, a novel acidic coiled-coil protein of the Dictyostelium centrosome, affects growth, chemotaxis, and the nuclear envelope. Mol Biol Cell 20:4348–4361
Kuhnert O, Baumann O, Meyer I, Gräf R (2012) Functional characterization of CP148, a novel key component for centrosome integrity in Dictyostelium. Cell Mol Life Sci. 69:1875–1888
Samereier M, Baumann O, Meyer I, Gräf R (2011) Analysis of Dictyostelium TACC reveals differential interactions with CP224 and unusual dynamics of Dictyostelium microtubules. Cell Mol Life Sci 68:275–287
Gräf R (2002) DdNek2, the first non-vertebrate homologue of human Nek2, is involved in the formation of microtubule-organizing centers. J Cell Sci 115:1919–1929
Reinders Y, Schulz I, Gräf R, Sickmann A (2006) Identification of novel centrosomal proteins in Dictyostelium discoideum by comparative proteomic approaches. J Proteome Res 5:589–598
Schulz I, Baumann O, Samereier M, Zoglmeier C, Gräf R (2009) Dictyostelium Sun1 is a dynamic membrane protein of both nuclear membranes and required for centrosomal association with clustered centromeres. Eur J Cell Biol 88:621–638
Krüger A, Batsios P, Baumann O, Luckert E, Schwarz H, Stick R, Meyer I, Gräf R (2012) Characterization of NE81, the first lamin-like nucleoskeleton protein in a unicellular organism. Mol Biol Cell 23:360–370
Ding R, West RR, Morphew M, Oakley BR, McIntosh JR (1997) The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell 8:1461–1479
Ueda M, Schliwa M, Euteneuer U (1999) Unusual centrosome cycle in Dictyostelium: correlation of dynamic behavior and structural changes. Mol Biol Cell 10:151–160
Moens PB (1976) Spindle and kinetochore morphology of Dictyostelium discoideum. J Cell Biol 68:113–122
Rivero F, Maniak M (2006) Quantitative and microscopic methods for studying the endocytic pathway. Methods Mol Biol 346:423–438
Weiner OH, Murphy J, Griffiths G, Schleicher M, Noegel AA (1993) The actin-binding protein comitin (p24) is a component of the golgi apparatus. J Cell Biol 123:23–34
Gräf R, Daunderer C, Schliwa M (1999) Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol Cell 91:471–477
Lefkir Y, de Chassey B, Dubois A, Bogdanovic A, Brady RJ, Destaing O, Bruckert F, O’Halloran TJ, Cosson P, Letourneur F (2003) The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells. Mol Biol Cell 14:1835–1851
Ma S, Triviños Lagos L, Gräf R, Chisholm RL (1999) Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J Cell Biol 147:1261–1274
Kitanishi T, Shibaoka H, Fukui Y (1984) Disruption of microtubules and retardation of development of Dictyostelium with Ethyl N-phenylcarbamate and Thiabendazole. Protoplasma 120:185–196
Kastner PM, Schleicher M, Müller-Taubenberger A (2011) The NDR family kinase NdrA of Dictyostelium localizes to the centrosome and is required for efficient phagocytosis. Traffic 12:301–312
Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G (1995) Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein tag. Cell 83:915–924
Gerisch G, Benjak A, Köhler J, Weber I, Schneider N (2004) GFP-golvesin constructs to study Golgi tubulation and post-Golgi vesicle dynamics in phagocytosis. Eur J Cell Biol 83:297–303
Samereier M, Meyer I, Koonce MP, Gräf R (2010) Live cell-imaging techniques for analyses of microtubules in Dictyostelium. Methods Cell Biol 97:341–357
Fukui Y, Yumura S, Yumura TK (1987) Agar-overlay immunofluorescence: high resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol 28:347–356
Gräf R, Daunderer C, Schliwa M (2000) Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J Cell Sci 113:1747–1758
Gräf R, Euteneuer U, Ueda M, Schliwa M (1998) Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur J Cell Biol 76:167–175
Wehland J, Willingham MC (1983) A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. II. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of Golgi elements. J Cell Biol 97:1476–1490
Faix J, Kreppel L, Shaulsky G, Schleicher M, Kimmel AR (2004) A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res 32, e143
Acknowledgments
We would like to thank Belinda Pipke for technical assistance. We also acknowledge Prof. Dr. Michael Schleicher for providing the anti-comitin antibody and Dr. Alexandra Lepier for critically reading the manuscript. This work was supported by DFG GR1642/3-1 and GR1642/4-1.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2012_1040_MOESM1_ESM.jpg
Supplementary Fig. S1 The GFP-CP55 fusion protein localizes to the centrosome during the entire cell cycle. GFP-CP55 cells were fixed with glutaraldehyde and stained with anti-α-tubulin YL1/2 [38]/AlexaFluor 568 anti-rat IgG (red) and DAPI (blue); GFP fluorescence is shown in green. Cell cycle stages and stainings are indicated. Maximum intensity projections of deconvolved wide field image stacks are shown. Bar = 3 μm. (JPEG 247 kb)
18_2012_1040_MOESM2_ESM.jpg
Supplementary Fig. S2 CP55null cells are rescued by expression of GFP-CP55. There are no supernumerary MTOCs and there are no aberrant nuclei anymore. CP55null cells expressing GFP-CP55 were fixed with glutaraldehyde and stained with anti-α-tubulin YL1/2 [38]/AlexaFluor 568 anti-rat IgG (red) and DAPI (blue); GFP fluorescence is shown in green. Maximum intensity projections of deconvolved wide field image stacks are shown. Bar = 3 μm. (JPEG 380 kb)
18_2012_1040_MOESM3_ESM.jpg
Supplementary Fig. S3 CP55null cells are unable to grow on bacteria. CP55null cells and control cells were cultivated either in bacterial suspension in phosphate buffer (a, a') or on phosphate agar plates with a lawn of bacteria (b, b'). The behavior of CP55null cells and control cells was independent of the bacterial species, i.e. K. aerogenes (a, b) or E. coli (a', b'). (a, a') shows no clearing of the bacterial suspension in case of CP55null cells, while the parallel culture of control cells shows complete clearing of the bacterial suspension within a cultivation period of 3 days (72 hours). In (b, b') a slight clearing appeared where CP55null cells were applied, indicating uptake of bacteria, however the diameter of the clearing fitted to the diameter of the applied droplet of Dictyostelium cells and did not change evem within 24 days indicating that cells were unable to utilize the phagocytosed bacteria. The photo shows the situation after 4 days, when the clearing of control cells had grown considerably and fruiting bodies developed where bacteria were eaten up. (JPEG 791 kb)
18_2012_1040_MOESM4_ESM.mov
Movie S1 GFP-CP55 shows only little recovery in FRAP experiments. The movie pauses at the two bleaching events with a point-focused 473-nm laser pulse. Control cells in the same field of view were unaffected by the bleach. Confocal spinning disk microscopy at a frame rate of 7.5 fr/s without time lapse; maximum intensity projections of 5 slices per image stack are shown. (MOV 9,126 kb)
18_2012_1040_MOESM5_ESM.mov
Movie S2 CP55null cells are defective in progression through prophase and spindle formation. A binucleated cell is shown from prophase to cytokinesis. (Such binucleated cells are common, when Dictyostelium amoebae are grown in axenic medium). Prophase lasted for more than 35 min (2100 s). In this case centrosomal splitting in prometaphase occurred at both centrosomes, but only the upper bipolar spindle behaved normally. The lower one disintegrated in metaphase at time point 2300 s. In telophase (assessed by the distance of the poles in the intact spindle) supernumerary MTOCs arose through disintegration of the defect spindle (time points 2,500–2,800 s). Cytokinesis started at time point 3,120 s and ended with the formation of a small daughter cell. (Late failures of spindle formation, i.e. only in metaphase, are a rare event.) This example was chosen because here, formation of a normal, bipolar spindle and failure of spindle formation was visible in the same cell, and the time point of supernumerary MTOC formation was well illustrated through mitotic progression of the normal, bipolar spindle. (MOV 18,171 kb)
18_2012_1040_MOESM6_ESM.mov
Movie S3 CP55null cells are defective in progression through prophase and spindle formation. CP55null/GFP-α-Tubulin cell in mitosis from late prophase to telophase. The initial centrosome/spindle pole is marked by an asterisk. A monopolar spindle was formed, and a metaphase-like stage was reached only at 1,100 s. Note the freely moving distal end of the monopolar spindle. Starting at time point 1260 s, severing of individual microtubules was discernible, which became part of new MTOCs. Cytokinesis started at time point 1,360 s and led to a tripartite cell. However, cytokinesis was not completed, and the already separated parts of the cell fused again resulting in a cell containing several MTOCs. Since the cell was moving out of the field of view, it had to be repositioned several times during image acquisition, which is indicated by insertion of a black image. (MOV 26,100 kb)
18_2012_1040_MOESM7_ESM.mov
Movie S4 Knockout of CP55 does not interfere with phagocytosis of yeast cells. The movie shows ingestion of a TRITC-labeled yeast cell by CP55null cells expressing GFP-LIMΔcoil as a marker of F-actin in phagocytic cups. Confocal spinning disk microscopy with a time interval of 10 s per image stack; maximum intensity projections of 7 slices per image stack recorded at a frame rate of 7 fr/s are shown. Bar 3 μm (MOV 1,640 kb)
Rights and permissions
About this article
Cite this article
Kuhnert, O., Baumann, O., Meyer, I. et al. CP55, a novel key component of centrosomal organization in Dictyostelium . Cell. Mol. Life Sci. 69, 3651–3664 (2012). https://doi.org/10.1007/s00018-012-1040-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1040-3