Abstract
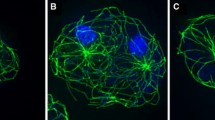
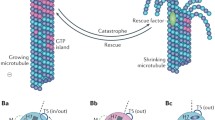
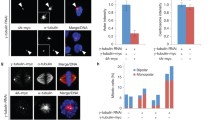
We have localized TACC to the microtubule-nucleating centrosomal corona and to microtubule plus ends. Using RNAi we proved that Dictyostelium TACC promotes microtubule growth during interphase and mitosis. For the first time we show in vivo that both TACC and XMAP215 family proteins can be differentially localized to microtubule plus ends during interphase and mitosis and that TACC is mainly required for recruitment of an XMAP215-family protein to interphase microtubule plus ends but not for recruitment to centrosomes and kinetochores. Moreover, we have now a marker to study dynamics and behavior of microtubule plus ends in living Dictyostelium cells. In a combination of live cell imaging of microtubule plus ends and fluorescence recovery after photobleaching (FRAP) experiments of GFP-α-tubulin cells we show that Dictyostelium microtubules are dynamic only in the cell periphery, while they remain stable at the centrosome, which also appears to harbor a dynamic pool of tubulin dimers.













Similar content being viewed by others
Abbreviations
- FRAP:
-
Fluorescence recovery after photobleaching
- MAP:
-
Microtubule-associated protein
- TBZ:
-
Thiabendazole
References
Dammermann A, Desai A, Oegema K (2003) The minus end in sight. Curr Biol 13:R614–R624
Carvalho P, Tirnauer JS, Pellman D (2003) Surfing on microtubule ends. Trends Cell Biol 13:229–237
Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, Vale RD (2005) Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J Cell Biol 168:587–598
Gräf R, Daunderer C, Schliwa M (2000) Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J Cell Sci 113:1747–1758
Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA (2008) XMAP215 is a processive microtubule polymerase. Cell 132:79–88
Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW (2001) Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behavior. Nat Cell Biol 3:643–649
Gard DL, Becker BE, Josh Romney S (2004) MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins. Int Rev Cytol 239:179–272
Gard DL, Kirschner MW (1987) A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol 105:2203–2215
Rehberg M, Gräf R (2002) Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol Biol Cell 13:2301–2310
Hestermann A, Gräf R (2004) The XMAP215-family protein DdCP224 is required for cortical interactions of microtubules. BMC Cell Biol 5:24
Gräf R, Euteneuer U, Ho TH, Rehberg M (2003) Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol Biol Cell 14:4067–4074
Koch KV, Reinders Y, Ho TH, Sickmann A, Gräf R (2006) Identification and isolation of Dictyostelium microtubule-associated protein interactors by tandem affinity purification. Eur J Cell Biol 85:1079–1090
Still IH, Hamilton M, Vince P, Wolfman A, Cowell JK (1999) Cloning of TACC1, an embryonically expressed, potentially transforming coiled coil containing gene, from the 8p11 breast cancer amplicon. Oncogene 18:4032–4038
Gergely F (2002) Centrosomal TACCtics. Bioessays 24:915–925
Peset I, Vernos I (2008) The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol 18:379–388
Gergely F, Karlsson C, Still I, Cowell J, Kilmartin J, Raff JW (2000) The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc Natl Acad Sci USA 97:14352–14357
Gergely F, Kidd D, Jeffers K, Wakefield JG, Raff JW (2000) D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J 19:241–252
Le Bot N, Tsai MC, Andrews RK, Ahringer J (2003) TAC-1, a regulator of microtubule length in the C. elegans embryo. Curr Biol 13:1499–1505
Peset I, Seiler J, Sardon T, Bejarano LA, Rybina S, Vernos I (2005) Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J Cell Biol 170:1057–1066
Cullen CF, Ohkura H (2001) Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat Cell Biol 3:637–642
Bellanger JM, Gonczy P (2003) TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr Biol 13:1488–1498
Srayko M, Quintin S, Schwager A, Hyman AA (2003) Caenorhabditis elegans TAC-1 and ZYG-9 form a complex that is essential for long astral and spindle microtubules. Curr Biol 13:1506–1511
Kinoshita K, Noetzel TL, Arnal I, Drechsel DN, Hyman AA (2006) Global and local control of microtubule destabilization promoted by a catastrophe kinesin MCAK/XKCM1. J Muscle Res Cell Motil 27:107–114
Albee AJ, Wiese C (2008) Xenopus TACC3/maskin is not required for microtubule stability but is required for anchoring microtubules at the centrosome. Mol Biol Cell 19:3347–3356
Schulz I, Baumann O, Samereier M, Zoglmeier C, Gräf R (2009) Dictyostelium Sun1 is a dynamic membrane protein of both nuclear membranes and required for centrosomal association with clustered centromeres. Eur J Cell Biol 88:621–638
Martens H, Novotny J, Oberstrass J, Steck TL, Postlethwait P, Nellen W (2002) RNAi in Dictyostelium: the role of RNA-directed RNA polymerases and double-stranded RNase. Mol Biol Cell 13:445–453
Bretschneider T, Anderson K, Ecke M, Muller-Taubenberger A, Schroth-Diez B, Ishikawa-Ankerhold HC, Gerisch G (2009) The three-dimensional dynamics of actin waves, a model of cytoskeletal self-organization. Biophys J 96:2888–2900
Müller-Taubenberger A, Vos MJ, Bottger A, Lasi M, Lai FP, Fischer M, Rottner K (2006) Monomeric red fluorescent protein variants used for imaging studies in different species. Eur J Cell Biol 85:1119–1129
Samereier M, Meyer I, Koonce MP, Gräf R (2010) Live cell imagine techniques for analyses of microtubules in Dictyostelium. Methods Cell Biol (in press)
Fukui Y, Yumura S, Yumura TK (1987) Agar-overlay immunofluorescence: high resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol 28:347–356
Gräf R, Euteneuer U, Ueda M, Schliwa M (1998) Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur J Cell Biol 76:167–175
Daunderer C, Gräf R (2002) Molecular analysis of the cytosolic Dictyostelium γ-tubulin complex. Eur J Cell Biol 81:175–184
Gräf R, Daunderer C, Schliwa M (1999) Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol Cell 91:471–477
Wehland J, Willingham MC (1983) A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin II. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of Golgi elements. J Cell Biol 97:1476–1490
Schulz I, Erle A, Gräf R, Kruger A, Lohmeier H, Putzler S, Samereier M, Weidenthaler S (2009) Identification and cell cycle-dependent localization of nine novel, genuine centrosomal components in Dictyostelium discoideum. Cell Motil Cytoskeleton 66:915–928
Brito DA, Strauss J, Magidson V, Tikhonenko I, Khodjakov A, Koonce MP (2005) Pushing forces drive the comet-like motility of microtubule arrays in Dictyostelium. Mol Biol Cell 16:3334–3340
Kimble M, Kuzmiak C, McGovern KN, de Hostos EL (2000) Microtubule organization and the effects of GFP-tubulin expression in Dictyostelium discoideum. Cell Motil Cytoskeleton 47:48–62
Koonce MP, Khodjakov A (2002) Dynamic microtubules in Dictyostelium. J Muscle Res Cell Motil 23:613–619
Acknowledgments
We would like to thank Dr. Katrin Pfütze for her engagement in the early stages of this project. We also acknowledge Anita Guhlan and Anne Krumbiegel for technical assistance. We are grateful to Dr. Annette Müller-Taubenberger (University of Munich, Institute for Cell Biology, Munich, Germany) for the marsRFP plasmid. This work was supported by the Deutsche Forschungsgemeinschaft GR1642/2-2.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Centrosomal dynamics of GFP-α-tubulin under the influence of the microtubule depolymerizing drug thiabendazole.A representative experiment of fluorescence recovery after photobleaching of centrosomal GFP-α-tubulin after incubation with 100 µM TBZ for 3 h is shown (the bleached region of interest was placed around the centrosome followed by 4D confocal live cell imaging. Fluorescence intensities in the bleached region of interest were evaluated with ImageJ. The half time of recovery and is indicated on the lower right (TIFF 14702 kb)
Fig. S2
Dynamic behavior of individual microtubules in the cell periphery of cells expressing marsRFP-α-tubulin and GFP-TACCdom.Two still images of movie 5 are shown. Tips of individual microtubules where movements are traceable are labeled with arrowheads (TIFF 23623 kb)
Movie 1
For FRAP analysis of centrosomal GFP-CP224 during interphase the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of 5 images with an interval of 0,5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 721 kb)
Movie 2
For FRAP analysis of centrosomal GFP-CP224 in TACC depleted cells during interphase the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of 5 images with an interval of 0,5 μm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 481 kb)
Movie 3
For FRAP analysis of spindle pole GFP-CP224 during mitosis the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 512 kb)
Movie 4
For FRAP analysis of spindle pole GFP-CP224 in TACC depleted cells during mitosis the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of 5 images with an interval of 0,5 μm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 1926 kb)
Movie 5
To study microtubule plus-end dynamics in vivo, a time series of confocal images of cells expressing marsRFP-α-tubulin and GFP-TACCdom as a microtubule plus-end marker were taken during interphase. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left(MPG 6434 kb)
Movie 6
For FRAP analysis of GFP-α-tubulin at microtubule plus ends the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 2816 kb)
Movie 7
For FRAP analysis of centrosomal GFP-α-tubulin during interphase the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 5248 kb)
Movie 8
For FRAP analysis of centrosomal GFP-α-tubulin in TACC depleted cells during interphase the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 4994 kb)
Movie 9
For FRAP analysis of spindle pole GFP-α-tubulin during mitosis the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 2492 kb)
Movie 10
For FRAP analysis of spindle pole GFP-α-tubulin in TACC depleted cells during mitosis the framed area was bleached followed by 4D confocal live cell imaging. For each time point, a z-stack consisting of five images with an interval of 0.5 µm was taken. Shown is a maximum intensity projection. The time interval is indicated on the upper left (MPG 3454 kb)
Rights and permissions
About this article
Cite this article
Samereier, M., Baumann, O., Meyer, I. et al. Analysis of Dictyostelium TACC reveals differential interactions with CP224 and unusual dynamics of Dictyostelium microtubules. Cell. Mol. Life Sci. 68, 275–287 (2011). https://doi.org/10.1007/s00018-010-0453-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0453-0