Abstract
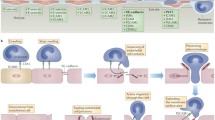
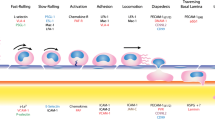
Leukocyte trafficking from the bloodstream to inflamed tissues across the endothelial barrier is an essential response in innate immunity. Leukocyte adhesion, locomotion, and diapedesis induce signaling in endothelial cells and this is accompanied by a profound reorganization of the endothelial cell surfaces that is only starting to be unveiled. Here we review the current knowledge on the leukocyte-mediated alterations of endothelial membrane dynamics and their role in promoting leukocyte extravasation. The formation of protein- and lipid-mediated cell adhesion nanodomains at the endothelial apical surface, the extension of micrometric apical membrane docking structures, which are derived from microvilli and embrace adhered leukocytes, as well as the vesicle-trafficking pathways that are required for efficient leukocyte diapedesis, are discussed. The coordination between these different endothelial membrane-remodeling events probably provides the road map for transmigrating leukocytes to find exit points in the vessel wall, in a context of severe mechanical and inflammatory stress. A better understanding of how vascular endothelial cells respond to immune cell adhesion should enable new therapeutic strategies to be developed that can abrogate uncontrolled leukocyte extravasation in inflammatory diseases.



Similar content being viewed by others
Abbreviations
- PMN:
-
Polymorphonuclear
- FCS:
-
Fluorescence correlation spectroscopy
- TIRF:
-
Total internal reflection fluorescence
- FRET:
-
Fluorescent resonance energy transfer
- FRAP:
-
Fluorescence recovery after photobleaching
- TEM:
-
Transendothelial migration
- BBB:
-
Blood–brain barrier
- LBR:
-
Lateral border recycling
- DRM:
-
Detergent-resistant membranes
References
Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7(10):803–815
Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109(23 Suppl 1):III27–III32
Butcher EC (1991) Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67(6):1033–1036
Libby P (2002) Inflammation in atherosclerosis. Nature 420(6917):868–874
McMurray RW (1996) Adhesion molecules in autoimmune disease. Semin Arthritis Rheum 25(4):215–233
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517
Nourshargh S, Hordijk PL, Sixt M (2010) Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol 11(5):366–378. doi:10.1038/nrm2889
Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD (1993) Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 74(3):541–554
Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD (1996) Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 84(4):563–574
Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML et al (1994) Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity 1(8):709–720
Oppenheimer-Marks N, Davis LS, Bogue DT, Ramberg J, Lipsky PE (1991) Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol 147(9):2913–2921
Jones DA, McIntire LV, Smith CW, Picker LJ (1994) A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J Clin Invest 94(6):2443–2450
Bowden RA, Ding ZM, Donnachie EM, Petersen TK, Michael LH, Ballantyne CM, Burns AR (2002) Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res 90(5):562–569
Bochner BS, Luscinskas FW, Gimbrone MA Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP (1991) Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med 173(6):1553–1557
Nandi A, Estess P, Siegelman M (2004) Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity 20(4):455–465
Zarbock A, Ley K (2009) New insights into leukocyte recruitment by intravital microscopy. Curr Top Microbiol Immunol 334:129–152. doi:10.1007/978-3-540-93864-4_6
Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7(9):678–689. doi:10.1038/nri2156
Millan J, Ridley AJ (2005) Rho GTPases and leucocyte-induced endothelial remodelling. Biochem J 385(Pt 2):329–337
Muller WA (2003) Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 24(6):327–334
Weber C, Fraemohs L, Dejana E (2007) The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol 7(6):467–477
Zarbock A, Ley K, McEver RP, Hidalgo A (2011) Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118(26):6743–6751. doi:10.1182/blood-2011-07-343566
Barreiro O, de la Fuente H, Mittelbrunn M, Sanchez-Madrid F (2007) Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev 218:147–164
Schenkel AR, Mamdouh Z, Muller WA (2004) Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol 5(4):393–400
Wittchen ES (2009) Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci 14:2522–2545
Vestweber D, Broermann A, Schulte D (2010) Control of endothelial barrier function by regulating vascular endothelial-cadherin. Curr Opin Hematol 17(3):230–236. doi:10.1097/MOH.0b013e328338664b
Vestweber D (2007) Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev 218:178–196. doi:10.1111/j.1600-065X.2007.00533.x
van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL (2003) VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol 285(2):C343–C352
Allingham MJ, van Buul JD, Burridge K (2007) ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 179(6):4053–4064
Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J (2008) Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 121(Pt 1):29–37
Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW (2005) ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-{alpha} activated vascular endothelium under flow. Blood 106(2):584–592
Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM (1995) IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol 154(12):6582–6592
Martinez-Estrada OM, Manzi L, Tonetti P, Dejana E, Bazzoni G (2005) Opposite effects of tumor necrosis factor and soluble fibronectin on junctional adhesion molecule-A in endothelial cells. Am J Physiol Lung Cell Mol Physiol 288(6):L1081–L1088
Fernandez-Borja M, van Buul JD, Hordijk PL (2010) The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc Res 86(2):202–210. doi:10.1093/cvr/cvq003
Shulman Z, Alon R (2012) Real-time analysis of integrin-dependent transendothelial migration and integrin-independent interstitial motility of leukocytes. Methods Mol Biol 757:31–45. doi:10.1007/978-1-61779-166-6_3
Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B (2003) T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood 102(10):3675–3683
Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, Adamson P (2003) Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol 171(4):2099–2108
Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA (2002) CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol 3(2):143–150
Rahman A, Fazal F (2009) Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal 11(4):823–839. doi:10.1089/ARS.2008.2204
Wang J, Springer TA (1998) Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol Rev 163:197–215
van Buul JD, van Rijssel J, van Alphen FP, van Stalborch AM, Mul EP, Hordijk PL (2010) ICAM-1 clustering on endothelial cells recruits VCAM-1. J Biomed Biotechnol 2010:120328. doi:10.1155/2010/120328
Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F (2008) Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol 183(3):527–542
Edwards S, Lalor PF, Nash GB, Rainger GE, Adams DH (2005) Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology 41(3):451–459. doi:10.1002/hep.20585
Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E (2009) Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J 420(2):133–154. doi:10.1042/BJ20082422
Stipp CS, Kolesnikova TV, Hemler ME (2003) Functional domains in tetraspanin proteins. Trends Biochem Sci 28(2):106–112
Kitadokoro K, Bordo D, Galli G, Petracca R, Falugi F, Abrignani S, Grandi G, Bolognesi M (2001) CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J 20(1–2):12–18. doi:10.1093/emboj/20.1.12
Yang X, Claas C, Kraeft SK, Chen LB, Wang Z, Kreidberg JA, Hemler ME (2002) Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol Biol Cell 13(3):767–781. doi:10.1091/mbc.01-05-0275
Espenel C, Margeat E, Dosset P, Arduise C, Le Grimellec C, Royer CA, Boucheix C, Rubinstein E, Milhiet PE (2008) Single-molecule analysis of CD9 dynamics and partitioning reveals multiple modes of interaction in the tetraspanin web. J Cell Biol 182(4):765–776. doi:10.1083/jcb.200803010
Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19(9):434–446. doi:10.1016/j.tcb.2009.06.004
Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11(10):688–699
Brown DA, Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68(3):533–544
Munro S (2003) Lipid rafts: elusive or illusive? Cell 115(4):377–388
Tilghman RW, Hoover RL (2002) E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Lett 525(1–3):83–87
Kiely JM, Hu Y, Garcia-Cardena G, Gimbrone MA Jr (2003) Lipid raft localization of cell surface E-selectin is required for ligation-induced activation of phospholipase C gamma. J Immunol 171(6):3216–3224
Lajoie P, Goetz JG, Dennis JW, Nabi IR (2009) Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol 185(3):381–385
Parton RG (2003) Caveolae—from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol 4(2):162–167
Chidlow JH Jr, Sessa WC (2010) Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 86(2):219–225
Lajoie P, Nabi IR (2010) Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol 282:135–163. doi:10.1016/S1937-6448(10)82003-9
Li XA, Everson WV, Smart EJ (2005) Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med 15(3):92–96
Setiadi H, McEver RP (2008) Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow. Blood 111(4):1989–1998. doi:10.1182/blood-2007-09-113423
Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ (2006) Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol 8(2):113–123
van Buul JD, van Rijssel J, van Alphen FP, Hoogenboezem M, Tol S, Hoeben KA, van Marle J, Mul EP, Hordijk PL (2010) Inside-out regulation of ICAM-1 dynamics in TNF-alpha-activated endothelium. PLoS One 5(6):e11336. doi:10.1371/journal.pone.0011336
Keuschnigg J, Henttinen T, Auvinen K, Karikoski M, Salmi M, Jalkanen S (2009) The prototype endothelial marker PAL-E is a leukocyte trafficking molecule. Blood 114(2):478–484
Carman CV, Springer TA (2004) A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol 167(2):377–388
Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martin-Belmonte F (2010) The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol 189(4):725–738. doi:10.1083/jcb.201002047
Xu C, Zhang YH, Thangavel M, Richardson MM, Liu L, Zhou B, Zheng Y, Ostrom RS, Zhang XA (2009) CD82 endocytosis and cholesterol-dependent reorganization of tetraspanin webs and lipid rafts. FASEB J 23(10):3273–3288. doi:10.1096/fj.08-123414
Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (2002) Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157(7):1233–1245
Hogue IB, Grover JR, Soheilian F, Nagashima K, Ono A (2011) Gag induces the coalescence of clustered lipid rafts and tetraspanin-enriched microdomains at HIV-1 assembly sites on the plasma membrane. J Virol 85(19):9749–9766. doi:10.1128/JVI.00743-11
Silvie O, Charrin S, Billard M, Franetich JF, Clark KL, van Gemert GJ, Sauerwein RW, Dautry F, Boucheix C, Mazier D, Rubinstein E (2006) Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci 119(Pt 10):1992–2002. doi:10.1242/jcs.02911
Zilber MT, Setterblad N, Vasselon T, Doliger C, Charron D, Mooney N, Gelin C (2005) MHC class II/CD38/CD9: a lipid-raft-dependent signaling complex in human monocytes. Blood 106(9):3074–3081. doi:10.1182/blood-2004-10-4094
Cherukuri A, Shoham T, Sohn HW, Levy S, Brooks S, Carter R, Pierce SK (2004) The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J Immunol 172(1):370–380
Lange K (2011) Fundamental role of microvilli in the main functions of differentiated cells: outline of an universal regulating and signaling system at the cell periphery. J Cell Physiol 226(4):896–927. doi:10.1002/jcp.22302
Diakowski W, Grzybek M, Sikorski AF (2006) Protein 4.1, a component of the erythrocyte membrane skeleton and its related homologue proteins forming the protein 4.1/FERM superfamily. Folia Histochem Cytobiol 44(4):231–248
Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O (1998) Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem 273(34):21893–21900
Oh HM, Lee S, Na BR, Wee H, Kim SH, Choi SC, Lee KM, Jun CD (2007) RKIKK motif in the intracellular domain is critical for spatial and dynamic organization of ICAM-1: functional implication for the leukocyte adhesion and transmigration. Mol Biol Cell 18(6):2322–2335
Ivetic A, Ridley AJ (2004) Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 112(2):165–176
Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11(4):276–287
Saotome I, Curto M, McClatchey AI (2004) Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6(6):855–864. doi:10.1016/j.devcel.2004.05.007
Yonemura S, Tsukita S, Tsukita S (1999) Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol 145(7):1497–1509
Wojciak-Stothard B, Williams L, Ridley AJ (1999) Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol 145(6):1293–1307
Carman CV, Jun CD, Salas A, Springer TA (2003) Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol 171(11):6135–6144
van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K (2007) RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol 178(7):1279–1293
Clancy RM, Abramson SB (2000) Acetylcholine prevents intercellular adhesion molecule 1 (CD54)-induced focal adhesion complex assembly in endothelial cells via a nitric oxide- cGMP-dependent pathway. Arthritis Rheum 43(10):2260–2264
Booth JW, Trimble WS, Grinstein S (2001) Membrane dynamics in phagocytosis. Semin Immunol 13(6):357–364
Heasman SJ, Ridley AJ (2008) Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9(9):690–701
Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16(10):522–529. doi:10.1016/j.tcb.2006.08.006
Martin-Belmonte F, Mostov K (2008) Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol 20(2):227–234. doi:10.1016/j.ceb.2008.01.001
Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128(2):383–397. doi:10.1016/j.cell.2006.11.051
Saito H, Minamiya Y, Saito S, Ogawa J (2002) Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol 72(4):829–836
Thompson PW, Randi AM, Ridley AJ (2002) Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol 169(2):1007–1013
Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO (1998) ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol 161(10):5755–5761
deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D, Hsu PK, Chou BK, Cheng LC, Blangy A, Sondek J, Hengartner MO, Wu YC, Ravichandran KS (2004) Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol 14(24):2208–2216. doi:10.1016/j.cub.2004.12.029
Cain RJ, Vanhaesebroeck B, Ridley AJ (2010) The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol 188(6):863–876
Katoh H, Negishi M (2003) RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 424(6947):461–464. doi:10.1038/nature01817
Katoh H, Hiramoto K, Negishi M (2006) Activation of Rac1 by RhoG regulates cell migration. J Cell Sci 119(Pt 1):56–65. doi:10.1242/jcs.02720
Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70(3):401–410
Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM (1997) Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol 138(6):1265–1278
Takenawa T, Suetsugu S (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8(1):37–48. doi:10.1038/nrm2069
Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW (2004) Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med 200(12):1571–1580
Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM (1998) Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med 187(6):903–915
Muller WA (2011) Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6:323–344. doi:10.1146/annurev-pathol-011110-130224
Holz LE, Warren A, Le Couteur DG, Bowen DG, Bertolino P (2010) CD8+ T cell tolerance following antigen recognition on hepatocytes. J Autoimmun 34(1):15–22. doi:10.1016/j.jaut.2009.08.005
Williamson JR, Grisham JW (1960) Leucocytic emigration from inflamed capillaries. Nature 188:1203
Williamson JR, Grisham JW (1961) Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol 39:239–256
Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R (2009) Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 30(3):384–396. doi:10.1016/j.immuni.2008.12.020
Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P (2006) Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203(12):2569–2575. doi:10.1084/jem.20060925
Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N (2005) A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol 170(1):141–151. doi:10.1083/jcb.200412032
Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA (2007) Transcellular diapedesis is initiated by invasive podosomes. Immunity 26(6):784–797
Carman CV, Springer TA (2008) Trans-cellular migration: cell–cell contacts get intimate. Curr Opin Cell Biol 20(5):533–540
Malsam J, Kreye S, Sollner TH (2008) Membrane fusion: SNAREs and regulation. Cell Mol Life Sci 65(18):2814–2832. doi:10.1007/s00018-008-8352-3
Lawson C, Wolf S (2009) ICAM-1 signaling in endothelial cells. Pharmacol Rep 61(1):22–32
van Buul JD, Kanters E, Hordijk PL (2007) Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol 27(9):1870–1876
Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO (2000) ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol 165(6):3375–3383
van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, van der Schoot CE, Hordijk PL (2002) Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol 168(2):588–596
Dejana E, Orsenigo F, Lampugnani MG (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121(Pt 13):2115–2122
Wang Q, Pfeiffer GR 2nd, Gaarde WA (2003) Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem 278(48):47731–47743
Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, Wolburg-Buchholz K, Wolburg H, Sorokin L, Zeuschner D, Maerz S, Butz S, Krombach F, Vestweber D (2010) CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 116(7):1172–1184. doi:10.1182/blood-2009-12-256388
Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, Vestweber D (2006) ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med 203(7):1671–1677. doi:10.1084/jem.20060565
Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M (2004) DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med 199(10):1331–1341
Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S (2007) JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood 110(6):1848–1856. doi:10.1182/blood-2006-09-047431
Aird WC (2003) Endothelial cell heterogeneity. Crit Care Med 31(4 Suppl):S221–S230
Garlanda C, Dejana E (1997) Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol 17(7):1193–1202
Albelda SM (1991) Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol 4(3):195–203
Dejana E (2004) Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol 5(4):261–270
Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA (2003) Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature 421(6924):748–753
Mamdouh Z, Kreitzer GE, Muller WA (2008) Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med 205(4):951–966. doi:10.1084/jem.20072328
Mamdouh Z, Mikhailov A, Muller WA (2009) Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med 206(12):2795–2808
Braet F, Riches J, Geerts W, Jahn KA, Wisse E, Frederik P (2009) Three-dimensional organization of fenestrae labyrinths in liver sinusoidal endothelial cells. Liver Int 29(4):603–613. doi:10.1111/j.1478-3231.2008.01836.x
Vasile E, Qu H, Dvorak HF, Dvorak AM (1999) Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J Histochem Cytochem 47(2):159–167
Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM (2004) Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J Histochem Cytochem 52(1):87–101
Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T (1999) Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol 163(2):553–557
Manes TD, Pober JS (2011) Identification of endothelial cell junctional proteins and lymphocyte receptors involved in transendothelial migration of human effector memory CD4+ T cells. J Immunol 186(3):1763–1768. doi:10.4049/jimmunol.1002835
Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C (2002) JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 3(2):151–158
Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT (2009) LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J 96(1):285–293. doi:10.1529/biophysj.108.135491
Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T (2002) The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med 196(5):679–691
Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA (2004) JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell 15(8):3926–3937. doi:10.1091/mbc.E04-04-0317
Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M (2005) Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell 16(10):4992–5003. doi:10.1091/mbc.E05-04-0310
Ludwig RJ, Hardt K, Hatting M, Bistrian R, Diehl S, Radeke HH, Podda M, Schon MP, Kaufmann R, Henschler R, Pfeilschifter JM, Santoso S, Boehncke WH (2009) Junctional adhesion molecule (JAM)-B supports lymphocyte rolling and adhesion through interaction with alpha4beta1 integrin. Immunology 128(2):196–205. doi:10.1111/j.1365-2567.2009.03100.x
Marmon S, Hinchey J, Oh P, Cammer M, de Almeida CJ, Gunther L, Raine CS, Lisanti MP (2009) Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am J Pathol 174(2):684–692. doi:10.2353/ajpath.2009.080091
Stan RV, Ghitescu L, Jacobson BS, Palade GE (1999) Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein. J Cell Biol 145(6):1189–1198
Stan RV, Kubitza M, Palade GE (1999) PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA 96(23):13203–13207
Stan RV, Tkachenko E, Niesman IR (2004) PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol Biol Cell 15(8):3615–3630. doi:10.1091/mbc.E03-08-0593
Stan RV (2007) Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol Med 11(4):621–643. doi:10.1111/j.1582-4934.2007.00075.x
Predescu SA, Predescu DN, Palade GE (2001) Endothelial transcytotic machinery involves supramolecular protein–lipid complexes. Mol Biol Cell 12(4):1019–1033
Pol A, Lu A, Pons M, Peiro S, Enrich C (2000) Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem 275(39):30566–30572. doi:10.1074/jbc.M001131200
Predescu SA, Predescu DN, Shimizu K, Klein IK, Malik AB (2005) Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J Biol Chem 280(44):37130–37138. doi:10.1074/jbc.M505659200
Schnitzer JE, Allard J, Oh P (1995) NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol 268(1 Pt 2):H48–H55
Dvorak AM, Feng D (2001) The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem 49(4):419–432
Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD (2008) Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res 102(12):e120–e131. doi:10.1161/CIRCRESAHA.107.167486
Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, Correas I, Ridley AJ (2010) Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol 8:11
Stahlhut M, van Deurs B (2000) Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell 11(1):325–337
Muriel O, Echarri A, Hellriegel C, Pavon DM, Beccari L, Del Pozo MA (2011) Phosphorylated filamin A regulates actin-linked caveolae dynamics. J Cell Sci 124(Pt 16):2763–2776. doi:10.1242/jcs.080804
Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD (2009) Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 20(21):4531–4540. doi:10.1091/mbc.E08-10-0997
Kanters E, van Rijssel J, Hensbergen PJ, Hondius D, Mul FP, Deelder AM, Sonnenberg A, van Buul JD, Hordijk PL (2008) Filamin B mediates ICAM-1-driven leukocyte transendothelial migration. J Biol Chem 283(46):31830–31839
Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW (2006) Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res 98(3):394–402. doi:10.1161/01.RES.0000201958.59020.1a
Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW (2006) Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol 177(9):6440–6449
Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D (2011) Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med 208(8):1721–1735. doi:10.1084/jem.20101920
Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X (2001) Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol 3(3):259–266. doi:10.1038/35060051
Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP (2001) Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol 155(4):511–517
Viola A, Gupta N (2007) Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol 7(11):889–896
Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, Klein E, Shinder V, Stoler-Barak L, Feigelson SW, Meshel T, Nurmi SM, Goldstein I, Hartley O, Gahmberg CG, Etzioni A, Weninger W, Ben-Baruch A, Alon R (2011) Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol. doi:10.1038/ni.2173
Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD (2009) Introducing intermediate filaments: from discovery to disease. J Clin Investig 119(7):1763–1771. doi:10.1172/JCI38339
Pallari HM, Eriksson JE (2006) Intermediate filaments as signaling platforms. Sci STKE 2006(366):pe53. doi:10.1126/stke.3662006pe53
Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S (2006) Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol 8(2):156–162
Sprenger RR, Fontijn RD, van Marle J, Pannekoek H, Horrevoets AJ (2006) Spatial segregation of transport and signalling functions between human endothelial caveolae and lipid raft proteomes. Biochem J 400(3):401–410. doi:10.1042/BJ20060355
Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S (2011) The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12(8):761–769. doi:10.1038/ni.2062
Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P (2008) Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One 3(2):e1649. doi:10.1371/journal.pone.0001649
Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D (2011) Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J 30(20):4157–4170. doi:10.1038/emboj.2011.304
Coisne C, Engelhardt B (2011) Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal 15(5):1285–1303. doi:10.1089/ars.2011.3929
Wolburg H, Wolburg-Buchholz K, Engelhardt B (2005) Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol 109(2):181–190. doi:10.1007/s00401-004-0928-x
Petri B, Kaur J, Long EM, Li H, Parsons SA, Butz S, Phillipson M, Vestweber D, Patel KD, Robbins SM, Kubes P (2011) Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood 117(3):942–952. doi:10.1182/blood-2010-02-270561
Wang HX, Kolesnikova TV, Denison C, Gygi SP, Hemler ME (2011) The C-terminal tail of tetraspanin protein CD9 contributes to its function and molecular organization. J Cell Sci 124(Pt 16):2702–2710. doi:10.1242/jcs.085449
Bari R, Guo Q, Xia B, Zhang YH, Giesert EE, Levy S, Zheng JJ, Zhang XA (2011) Tetraspanins regulate the protrusive activities of cell membrane. Biochem Biophys Res Commun 415(4):619–626. doi:10.1016/j.bbrc.2011.10.121
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G (2011) The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21(4):708–721. doi:10.1016/j.devcel.2011.08.019
Acknowledgments
We would like to thank M.A. Alonso, E. Cernuda-Morollón and S. Gharbi for critical reading of the manuscript. We also appreciate the help of F. Belio with the design of the figures. Dr. J. Millán is supported by grants SAF2008-01936, SAF2011-22624 from Ministerio de Ciencia e Innovación, Spain, and received research support from Biogen-Idec. B. Marcos-Ramiro is supported by an FPI fellowship from Ministerio de Ciencia e Innovación. N. Reglero-Real is supported by a JAE fellowship from CSIC.
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Reglero-Real and B. Marcos-Ramiro contributed equally to this work.
Rights and permissions
About this article
Cite this article
Reglero-Real, N., Marcos-Ramiro, B. & Millán, J. Endothelial membrane reorganization during leukocyte extravasation. Cell. Mol. Life Sci. 69, 3079–3099 (2012). https://doi.org/10.1007/s00018-012-0987-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-0987-4