Abstract
The LIM domain only 4 (LMO4) protein is expressed in the hypothalamus, but its function there is not known. Using mice with LMO4 ablated in postnatal glutamatergic neurons, including most neurons of the paraventricular (PVN) and ventromedial (VMH) hypothalamic nuclei where LMO4 is expressed, we asked whether LMO4 is required for metabolic homeostasis. LMO4 mutant mice exhibited early onset adiposity. These mice had reduced energy expenditure and impaired thermogenesis together with reduced sympathetic outflow to adipose tissues. The peptide hormone leptin, produced from adipocytes, activates Jak/Stat3 signaling at the hypothalamus to control food intake, energy expenditure, and fat metabolism. Intracerebroventricular infusion of leptin suppressed feeding similarly in LMO4 mutant and control mice. However, leptin-induced fat loss was impaired and activation of Stat3 in the VMH was blunted in these mice. Thus, our study identifies LMO4 as a novel modulator of leptin function in selective hypothalamic nuclei to regulate fat metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a global pandemic associated with an increased risk of death from heart disease and stroke. Identifying genetic modifiers that contribute to obesity is therefore of considerable clinical interest. Discovery of the peptide hormone leptin [1] has led to a better understanding of the sophisticated system that regulates metabolism. Leptin is secreted from adipose tissue in a manner that is directly proportional to the degree of adiposity [2]. By binding receptors on neurons of various hypothalamic nuclei, leptin works in a feedback loop to control food intake, energy expenditure, and fat stores. Leptin signaling in the arcuate nucleus (ARC) controls food intake by activating the anorexic POMC neurons [3], and mice deficient in leptin receptors are hyperphagic and obese [4]. In addition, excitatory glutamatergic neurons from the VMH project to POMC neurons of the ARC to inhibit feeding [5]. In the VMH, leptin also regulates energy homeostasis and fat metabolism [6, 7]. Leptin increases sympathetic adrenergic tone to adipose tissue and promotes lipolysis [8]. Leptin increases parasympathetic tone that enhances the insulin-dependent inhibition of gluconeogenesis by the liver [9].
The leptin receptor is a member of the type I cytokine receptor family including the IL-6 receptors. Leptin binds to its receptor and activates the Janus kinase (Jak) protein kinase that phosphorylates and engages the signal transducer and activator of transcription-3 (Stat3) pathway [10]. Activation of Stat3, a transcription factor that modulates gene expression, is essential for leptin action in the hypothalamus [11]. Factors that modulate Stat3 signaling in the hypothalamus would be expected to influence leptin signaling and metabolic homeostasis.
The LIM domain only 4 (LMO4) protein is a cytoplasmic and nuclear cofactor that interacts with several signaling molecules and transcription factors to regulate gene expression [12–17]. It also interacts with Jak and augments Stat3 signaling in hepatocytes [15]. Our own work showed that LMO4 also contributes to Stat3 activation in neurons [12, 14]. Importantly, LMO4 is expressed in selected nuclei of the hypothalamus, including the VMH and PVN [18]. Thus, given its function in Jak/Stat3 signaling, we asked whether LMO4 contributes to central leptin control of metabolic homeostasis.
LMO4 is expressed in the central nervous system early during embryonic development [13] and germline ablation of LMO4 is lethal prior to birth [19]. To circumvent this problem, we created mice in which LMO4 is selectively ablated in postmitotic glutamatergic neurons after birth. Since the majority of neurons of the VMH and PVN of the hypothalamus are glutamatergic [20, 21] and glutamatergic efferents from these two nuclei are important in regulating feeding and autonomic and neuroendocrine functions [5, 22, 23], these mice enabled us to address the importance of LMO4 in leptin signaling and hypothalamic control of metabolism.
Materials and methods
Animal care
All procedures were approved by the animal care and use committee of the University of Ottawa. Animals were bred and maintained under a 12 h light and 12 h dark (1800–0600) cycle. Food (regular chow) and water were provided ad libitum unless specified.
Generation of CamK2αCre/LMO4flox mice
CamK2αCre/LMO4flox and LMO4flox littermate mice were genotyped and maintained on a CD-1 background as described [16].
Food intake
Mice were transferred to individual housing and habituated for 1 week. Daytime and nighttime food intake was calculated by averaging the food intake of each mouse over the 3 day experimental period.
MRI
Six-month-old littermate male mice anesthetized by i.p. injection of ketamine/xylazine/acepromazine were scanned in pairs, and the images were acquired using a multichannel wrist coil adapted to the 3T GE MRI scanner.
Acute and chronic intracerebroventricular (icv) leptin infusion
Two-month-old male mice were anesthetized. For acute perfusion, 1 μg/μl recombinant mouse leptin (Invitrogen, Cat #PMP0013) in sterile saline was stereotaxically infused (0.25 μl/min). For chronic infusion, an Alzet osmotic pump (Alzet, CA, USA; 1007D) was implanted subcutaneously and 10 μg leptin was infused (0.5 μl/h) over 7 days.
Leptin ELISA
Blood samples were collected between 1400 and 1600 h for plasma leptin determination using a mouse Leptin (Cat. # 90030) ELISA kit (Crystal Chem, IL, USA).
Norepinephrine turnover rate (NETO)
Eight-week-old male mice received two intraperitoneal injections of dl-a-methyl-p-tyrosine (sigma) at time 0 (250 mg/kg, 25 mg/ml) and 2 h (125 mg/kg, 2.5 mg/ml). At 4 h time, tissues were harvested and NETO was determined as described [24].
Indirect calorimetry
Mice were single-housed in metabolic chambers, oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured for 24 h using an Oxymax system with automatic temperature and light controls (Columbus Instruments, Columbus, OH). Temperature was maintained at 24°C with 12 h light/dark cycle.
Quantitative real-time RT-PCR (qPCR)
Total RNA from hypothalamus was extracted for qPCR using Taq DNA polymerase/cyber green PCR mix. All mRNA levels were normalized to actin. qPCR primers used included: LMO4: 5′-GGACCGCTTTCTGCTCTATG-3′ 5′-AAGCACCGCTATTCCCAAAT-3′; ACC: 5′-AGCAGATCCGCAGCTTGGT-3′ 5′-ACTTCTGCTCGCTGGGTGG-3′; FAS: 5′-GCTGGCATTCGTGATGGAGTCGT-3′ 5′-AGGCCACCAGTGATGATGTAACTCT-3′; Hsl: 5′-GACTCACCGCTGACTTCCT-3′ 5′-CTGTCTCGTTGCGTTTGTAG-3′; Atgl: 5′-TGGGTGACCATCTACCTTCC-3′ 5′-CCCAGTGAGAGGTTGTTTCG-3′; actin: 5′-GCTTCTTTGCAGCTCCTTCG-3′ 5′-CCTTCTGACCCATTCCCACC-3′; FAAH: 5′-ATGTGGATAGCCTGGCATTGTG-3′ 5′-CTGGAGTGGGCATGGTGTAGTT-3′; UCP1: 5′-AAAGTCCGCCTTCAGATC-3′ 5′-AGTTTCGGCAATCTTCT-3′.
In situ hybridization, immunofluorescence, and western blot
In situ hybridization was performed as described [25] using 300 nucleotides of DIG-UTP-labeled LMO4 exon 2 anti-sense RNA probes. For pStat3 immunofluorescence, anti-phosphorylated Tyr705-Stat3 primary antibody (cell signaling technologies) were incubated for 48 h at 4°C and detected with biotin-conjugated secondary antibody followed by cy3-conjugated streptavidin. Western immunoblots were performed as described [16] using actin (sigma), Akt, and phosphorylated Ser473-Akt antibodies (cell signaling technologies).
Thermogenesis study
For cold challenge, mice were fasted 16 h and housed individually, without food and a minimum amount of cage bedding at 4°C for 4 h. Rectal temperature was measured. For non-shivering thermogenesis, mice at room temperature were anesthetized using a ketamine/xylazine, and rectal core temperature was measured.
Statistical analysis
All results are expressed as mean ± SEM and analyzed using Statistical Package for the Social Sciences (SPSS). For between group comparisons, a two-tailed unpaired Student’s t test was used. Where appropriate, data were analyzed by ANOVA followed by LSD post hoc test to compare means between groups. P values of <0.05 were considered significant.
Results
Neuron-targeted ablation of LMO4 causes adult onset obesity
LMO4 is expressed in the cortex, hippocampus, and hypothalamic nuclei including the PVN and VMH, but not the ARC (Fig. 1a–c). Although our model (Camk2αCre/LMO4flox) does not specifically target hypothalamic nuclei, the use of a Cre-transgene activated in the first post-natal week in glutamatergic neurons enabled ablation of LMO4 in the majority of neurons in the PVN and VMH [20, 21] as shown by in situ hybridization (Fig. 1d). We further confirmed that ablation of LMO4 was neural-specific by PCR amplification of genomic DNA extracted from various tissues (Fig. 1e). qPCR of hypothalamic RNA extracts showed a 90% reduction in LMO4 mRNA (Fig. 1f). The overt phenotype of this ablation was adult onset obesity (Fig. 1g). Male CamK2αCre/LMO4flox mice fed regular chow starting at 3 weeks of age at the time of weaning developed significant obesity between 3 and 4 months of age (Fig. 1h).
Neuron-specific ablation of LMO4 leads to adult onset obesity in CamK2αCre/LMO4flox male mice. In situ hybridization of 14 μm cryosections revealed LMO4 mRNA expression in the PVN (a) and the VMH (a, b), but not in the ARC (b) in littermate control mice. Low power magnifications revealed LMO4 expression in cortex, hippocampus, amygdala, and hypothalamus of a littermate control (c) but not in the CamK2αCre/LMO4flox mouse (d) at 2 months of age. Scale bars 200 μm (a, b) and 400 μm (c, d). e PCR amplification across the floxed exon 2 of LMO4 of genomic DNA from various tissues from CamK2αCre/LMO4flox mice at weaning reveals selective ablation of LMO4 in neuronal tissues (asterisks). Diagram shows amplified unfloxed fragment is larger than the floxed allele. (WAT White adipose tissue, Thy thyroid, Sto stomach, S.C. spinal cord, Pan pancreas, Mus muscle, Lun lung, Liv liver, Kid kidney, S.I. small intestine, L.I. large intestine, Hyt hypothalamus, Hrt heart, Tes testicle, Ctx cortex, Ceb cerebellum, B.S. brain stem, BAT brown adipose tissue). f Quantitative RT-PCR from RNA purified from the hypothalamus shows a 90% reduction in LMO4 levels (*p < 0.05, n = 6 per group) in CamK2αCre/LMO4flox mice (a.u. arbitrary units, normalized to littermate control). g Male CamK2αCre/LMO4flox mice (KO) aged 4 months were obviously obese compared to littermate controls (WT). h The onset of obesity was significant after 12 weeks (asterisk indicates p < 0.05, n ≥ 12 per group). Black diamonds WT, open diamonds KO
Disregulated fat metabolism in CamK2αCre/LMO4flox mice
Even though at 2 months of age there was no significant difference in body weights between CamK2αCre/LMO4flox mice and littermate controls, excess fat stores (adiposity) were detected (Fig. 2a). There was nearly twice the amount of gonadal white adipose tissue (gWAT) and interscapular brown adipose tissue (BAT) in mutant mice at 2 and 4 months of age (Fig. 2a, b). MRI scans showed the increased prevalence of visceral and subcutaneous adiposity at 4 months (Fig. 2c). qPCR revealed dysregulated lipogenic and lipolytic gene expression in white adipose tissue and liver of the mutant mice as early as 1 month of age (Fig. 2d, e). Increased lipid droplet deposits were prevalent in WAT and BAT at 4 months (Fig. 2f).
Increased adiposity in CamK2αCre/LMO4flox mice. Gonadal white adipose tissue (gWAT) weights were increased at 2 months (a) and 4 months (b) of age in KO mice (n = 6 per group). Brown adipose tissue (BAT) weights were also increased at 4 months (b). c MRI revealed extensive adiposity (white) of KO mice compared to WT littermate at 6 months. Water and oil standards are shown for comparison (representative scan of three mice per group). qPCR measured lipogenic (ACC and FAS) and lipolytic (HSL and Atgl) gene expression in liver (d) and in gonadal WAT (e) at 1 month of age (n ≥ 4 per group). Significantly elevated levels were detected in gWAT of KO mice. f Paraffin sections (6 μm) of WAT and BAT were stained with hematoxylin and eosin and showed increased lipid droplet size at 4 months of age. Representative image of three mice per group; scale bar 100 μm. g Plasma leptin levels were significantly elevated in KO mice at 4 months of age (n = 8–11 mice per group). h Daytime and nighttime food intake averaged over 3 days was not different at 2 months but was elevated in 4 month old KO mice (n = 9–20 mice per group). i Locomotor activity, measured by autonomic beam break over a 24 h period, was similar between littermate KO and WT at 4 months of age. Indirect calorimetry indicates similar metabolic rate between littermate KO and WT at 4 months of age. j Oxygen consumption (VO2) adjusted to lean body mass and k respiratory exchange rates (VCO2/VO2) were measured per mouse for 24 h at 2 months of age when the body weight was not different between WT and KO mice (n ≥ 8 per group). Light phase VO2 was reduced in KO mice (*p < 0.05). Filled bars WT, open bars KO
Surprisingly, even though mutant mice had increased adiposity at 2 months of age, which would predict elevated leptin secretion, their plasma leptin levels were similar to littermate controls (Fig. 2g), and importantly, they were not hyperphagic at this age (Fig. 2h). To account for adiposity, the energy expenditure was compared at 2 months of age. Voluntary locomotor activity monitored by beam break was not different (Fig. 2i). However, whole-body oxygen consumption (VO2) measured by indirect calorimetry and adjusted to lean body mass [26] at 2 months was lower during the light phase in mutant mice (Fig. 2j), indicating reduced energy expenditure. No difference in fuel type utilization as determined by respiratory exchange rate (RER) was detected (Fig. 2k). By 4 months LMO4 mutant mice became hyperleptinemic (Fig. 2g) and hyperphagic, with signs of leptin resistance, i.e., the central leptin effect to suppress feeding behavior was lost and food intake was elevated by 50% (Fig. 2h). Reduced energy expenditure, together with the late-onset hyperphagia, likely contribute to the marked weight gain seen at this stage (Fig. 1h).
Reduced sympathetic tone to adipose tissue in LMO4 mutant mice
Sympathetic neural activity is important to control fat metabolism, energy homeostasis, and heat production among other physiological processes [8, 27, 28]. To determine whether reduced metabolic activity and adiposity observed at the age of 2 months when food intake was not increased were associated with reduced sympathetic activity, we used two approaches to measure sympathetic activity. First, we measured body temperature in response to cold challenge as an index of sympathetic activity to BAT. Cold challenge is known to increase sympathetic activity to augment heat production and maintain body temperature [27]. We observed impaired thermogenesis in CamK2αCre/LMO4flox mice when core temperatures was measured in conscious mice exposed to 4°C for 4 h (shivering and nonshivering BAT thermogenesis, Fig. 3a) or in anesthetized mice at room temperature (nonshivering BAT thermogenesis, Fig. 3b). In line with these observations, basal levels of thermogenic mitochondrial uncoupling protein 1 (UCP1) mRNA were reduced in BAT of LMO4 mutant mice (Fig. 3c). A second approach to measure sympathetic activity was the norepinephrine turnover (NETO) assay; an index of sympathetic activity to peripheral tissues that is extrapolated from the rate of decline in tissue NE content after inhibiting catecholamine synthesis. The NETO assay revealed a significantly lower sympathetic outflow to BAT and gWAT in LMO4 mutant mice compared to littermate controls (Fig. 3d). Together, these results provide compelling evidence that sympathetic outflow to adipose tissues was lower in LMO4 mutant mice.
Reduced sympathetic tone to adipose tissues in CamK2αCre/LMO4flox mice. Reduced thermogenic ability was observed in 2-month-old CamK2αCre/LMO4flox male mice upon cold challenge of conscious mice at 4°C (a) or anesthetized mice at room temperature (b) (n = 6 per group). c Quantitative RT-PCR revealed lower levels of uncoupling protein-1 (UCP1) mRNA in BAT of cold-challenged KO mice. d Norepinephrine turnover (NETO) assay revealed reduced sympathetic outflow to BAT and WAT in KO mice at 2 months of age (n = 8 per group). WT (black diamonds), KO (open diamonds)
Central leptin signaling in the VMH is impaired in LMO4 mutant mice
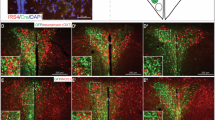
To test whether hypothalamic leptin signaling is affected by LMO4 ablation, leptin was injected into the cerebral ventricles and immunofluorescence was performed to compare phosphorylation of Stat3 in neurons of the VMH and ARC at 2 months of age, when endogenous plasma leptin levels are not different (see Fig. 2g). Markedly fewer phospho-Stat3-positive neurons were detected in the VMH of LMO4 mutant mice compared to littermate controls (Fig. 4a, c, e). In contrast, a similar number of phospho-Stat3-immunopositive neurons were detected in neurons in the ARC of littermate control and LMO4 mutant mice (Fig. 4b, d, e). Very few phospho-Stat3-immunopositive neurons were detected in the PVN (data not shown), consistent with the paucity of leptin-receptor-positive neurons in this nucleus [29]. These results show that LMO4 is required for leptin-induced Stat3 activation in the VMH but not in the ARC.
Selective reduction in central leptin signaling in CamK2αCre/LMO4flox mice. Immunofluorescence staining of phospho-Stat3 60 min after acute intracerebroventricular leptin injection revealed markedly reduced response in KO mice at the VMH, but a similar response in the ARC. a, b WT and c, d KO at 2 months of age. ARC Arcuate nucleus, VMH ventromedial hypothalamus. Scale bar 100 μm. Representative sections from leptin-injected mice are shown to illustrate different regions of the hypothalamus. e Quantification of pStat3-positive cells in different hypothalamic nuclei per section. Cells from both sides of hypothalamic nuclei between Bregma −1.6 and −1.8 mm were counted and averaged. Cells per section were counted for three to five sections from each mouse. n = 3 per group. Filled bars WT, open bars KO. *p < 0.05
Central leptin control of fat metabolism but not feeding behavior is impaired in LMO4 mutant mice
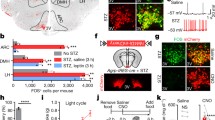
Hypothalamic leptin signaling causes weight loss by suppressing feeding and promoting lipolysis [8]. Chronic icv leptin infusion over 7 days (Fig. 5a) at 2 months of age suppressed feeding to a similar extent in both LMO4 mutant and littermate control mice (Fig. 5b). However, leptin-induced weight and fat loss were less pronounced in LMO4 mutant mice (Fig. 5c, d). One of the ways leptin induces fat loss is by increasing sympathetic outflow to adipose tissue to increase thermogenesis and energy expenditure. Central leptin infusion upregulated UCP1 mRNA in BAT of littermate control mice but failed to do so in LMO4 mutant mice (Fig. 5e). Thus, reduced thermogenesis and energy expenditure in LMO4 mutant mice after chronic leptin infusion would result in less fat loss.
Effects of chronic leptin infusion. a Diagram of the chronic icv leptin infusion protocol using an osmotic minipump in 2-month-old mice. Body weight changes were measured daily, and food intake was measured from day 4 to day 7 (n = 6–8 per group). b Leptin infusion suppressed food intake in both littermate control and KO mice. c Leptin-induced weight loss was not as great in KO mice as in WT mice (*p < 0.05). Black diamonds WT, open diamonds KO. d Leptin induced a 50% gonadal WAT (gWAT) weight loss in WT but not in KO mice. e Quantitative RT-PCR showed that leptin reduced lipogenic gene expression and activated the cannabinoid metabolizing enzyme FAAH in WAT of WT but not in KO mice. Similarly, leptin increased UCP1 mRNA expression in BAT in WT but not in KO mice
In addition, leptin-induced elevation of sympathetic activity activates the expression of fatty acid amidohydrolase (FAAH) in adipose tissue, and this would also promote fat loss [8]. FAAH is an enzyme that metabolizes the endogenous cannabinoid anandamide thereby preventing anandamide from activating CB1 receptors to promote lipogenesis [30]. Consequently, elevated FAAH favors lipolysis [8]. Chronic central leptin infusion increased FAAH expression in WAT of littermate control but not of LMO4 mutant mice, as revealed by qPCR (Fig. 5e). In line with this finding, expression of the lipogenic genes ACC and FAS was reduced in WAT of littermate control mice after leptin infusion, but not in LMO4 mutant mice (Fig. 5e). Taken together, these results demonstrate that LMO4 is important for hypothalamic leptin signaling to regulate fat metabolism.
Discussion
Our study identified LMO4 as a modulator of leptin signaling in the hypothalamus. Importantly, LMO4 is expressed in some, but not all, hypothalamic nuclei where leptin receptors are expressed [29]. We found that LMO4 is expressed in the VMH but not in the ARC, and consistent with their predominant effects in regulating fat metabolism/energy expenditure and feeding [31], respectively, ablation of LMO4 in the VMH caused a primary defect in the central control of fat metabolism rather than feeding behavior. This regional-specific expression pattern of LMO4 enables selective regulation of physiological activities that are controlled by hypothalamic leptin signaling.
Several lines of evidence support the requirement for LMO4 in modulating leptin signaling to control fat metabolism. First, leptin signaling phosphorylates and activates Stat3, but in the VMH of Camk2αCre/LMO4flox mice where LMO4 expression is ablated, leptin induced fewer phosphorylated-Stat3-immunopositive neurons compared to littermate control mice. Our finding that LMO4 is required for Jak/Stat3 signaling in VMH hypothalamic neurons is consistent with previous studies demonstrating that LMO4 participates in Jak/Stat3 signaling in hepatocytes [15] and in primary cortical neurons [14]. In hepatocytes, LMO4 was found to interact with Jak2, and ablation of LMO4 with siRNA blunted Stat3 phosphorylation and activation in response to IL-6 cytokine stimulation [15]. Thus, LMO4 modulation of Jak/Stat3 signaling is likely to be a general feature in tissues where LMO4 is expressed, including the VMH.
Second, leptin signaling in the VMH activates sympathetic outflow to adipose tissue to promote fat metabolism by inhibiting lipogenesis and increasing energy expenditure [7, 8, 32, 33]. Consistent with reduced leptin signaling in the VMH, sympathetic outflow to the WAT and BAT was significantly lower in LMO4 mutant mice. As early as 1 month, there was a tenfold higher expression of lipogenesis genes in WAT of LMO4 mutant mice. In the mutant mice, the BAT thermogenic response to cold challenge was impaired, the expression of thermogenic mitochondrial uncoupling protein UCP1 was lower and fat droplets were larger in BAT, indicative of reduced fat combustion. The consequent impaired thermogenic response and reduced oxygen consumption would result in an energy surplus and increased fat stores. Indeed, increased adiposity was observed at 2 months, when no increase in food intake was detected. Thus, impaired central leptin signaling and consequently lower sympathetic outflow likely account for the early appearance of adiposity in LMO4 mutant mice.
Third, fat loss induced by chronic central leptin infusion was impaired in LMO4 mutant mice. The anorexic response to central leptin administration, on the other hand, was relatively unaffected in LMO4 mutant mice. Unlike in the VMH, leptin induced a similar number of phospho-Stat3-positive neurons in the ARC, a key hypothalamic nucleus for feeding control. This result was not surprising since LMO4 is not expressed in the ARC. Taken together, these results highlight that the involvement of LMO4 in leptin-regulated neuroendocrine activities is highly selective, i.e. affecting fat metabolism rather than feeding suppression.
The phenotype of early increased adiposity without increased food intake observed in LMO4 mutant mice is reminiscent of mice with leptin receptors ablated in VMH neurons (SF1-Cre/Leprflox) [7, 34]. In these SF1-Cre/Leprflox mice, a significant expansion of both white and brown adipose compartments was noted, even when their weight and food intake [7] did not differ from WT mice, suggesting a defect in energy partitioning preferentially directing calories into fat rather than lean body mass [34]. However, adiposity in our mice becomes more pronounced than in SF-1Cre/Leprflox mice. Several factors are likely to contribute to this phenotype. First, early reduced energy expenditure likely accelerates energy surplus and fat accumulation in our mice. Second, LMO4 ablation in other brain regions in addition to the VMH, for example the PVN, is likely to worsen the obesity phenotype in our mice. Although few neurons in the PVN express leptin receptors [29], the PVN is a relay point receiving synaptic inputs from multiple hypothalamic nuclei to regulate energy expenditure and satiety [29, 35] and the PVN regulates sympathetic outflow to BAT and WAT [36]. In addition, the PVN contains neurons producing important neuropeptides such as oxytocin, corticotropin releasing hormone, and thyrotropin releasing hormone that play an important role in regulating food intake and energy expenditure [37–39]. Future studies will be needed to know which neuropeptides are expressed in LMO4-positive neurons of the PVN and how ablation of LMO4 in this nucleus affects metabolism. Impaired signaling through the PVN could also contribute to adiposity in the Camk2αCre/LMO4flox mice. Although outside the scope of the present study, the consequence of LMO4 ablation on synaptic transmission in the PVN may reveal additional mechanisms contributing to the metabolic defect in LMO4 mutant mice.
The onset of weight gain occurs much later in Camk2αCre/LMO4flox mice (around 12 weeks) compared to two similar mouse models that disrupt leptin signaling, Camk2αCre/LepRflox [40] and CamK2αCre/Shp2flox [41] mice, where the obesity phenotype is manifest at 5–6 weeks of age. We reasoned that early disruption of leptin signaling in the ARC in addition to other hypothalamic nuclei might account for the earlier weight gain in these two mouse models. As CamK2αCre transgene is expressed in the ARC, leptin signaling is disrupted in the ARC of Camk2αCre/LepRflox [40] and CamK2αCre/Shp2flox [41] mice early after birth. In contrast, because LMO4 is not expressed in the ARC, leptin signaling remains intact in the ARC of LMO4 mutant mice at least at 2 months of age. As adiposity progresses in the LMO4 mutant mice, they become hyperleptinemic and hyperphagic at 4 months of age, indicating that the response to leptin in the ARC becomes defective. It is well-established that hyperleptinemia is associated with hyperphagia and impaired leptin signaling in the ARC [42]. Hyperphagia would contribute to the accelerated weight gain observed at 4 months of age in LMO4 mutant mice.
Our findings are likely to be relevant to human obesity. Functional promoter polymorphisms that affect the level of LMO4 expression have been described [43]. Thus, differences in LMO4 expression in hypothalamic neurons might affect leptin signaling and susceptibility to obesity. In addition, LMO4 expression in the brain is dynamically regulated under different physiological conditions: it fluctuates with a circadian rhythm in the suprachiasmatic nucleus of the hypothalamus [44]; it responds to hyper- and hypo-osmotic stresses in the supraoptic nucleus of the hypothalamus [45], to sleep deprivation in the cerebral cortex [46], and to ischemic stress in striatal and cortical neurons [16]. Future studies will need to address whether physiological stresses influence fat metabolism by altering central LMO4 expression.
In summary, we present a novel mouse model of early onset adiposity progressing to adult obesity. Our study showed that LMO4 is a novel and regional-specific modulator of leptin signaling that enables selective leptin actions to control fat metabolism.
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1:1311–1314
Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K, Yoshimasa Y, Nishi S, Hosoda K, Nakao K (1997) The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett 224:149–152
Chua SC Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271:994–996
Sternson SM, Shepherd GM, Friedman JM (2005) Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci 8:1356–1363
Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB (2007) Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5:383–393
Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB (2006) Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203
Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L (2008) Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14:667–675
German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ (2009) Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150:4502–4511
Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC (1996) Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 93:6231–6235
Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG Jr (2004) LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53:3067–3073
Chen HH, Schock SC, Xu J, Safarpour F, Thompson CS, Stewart AF (2007) Extracellular ATP-dependent upregulation of the transcription cofactor LMO4 promotes neuron survival from hypoxia. Exp Cell Res 313:3106–3116
Chen HH, Yip JW, Stewart AF, Frank E (2002) Differential expression of a transcription regulatory factor, the LIM domain only 4 protein Lmo4, in muscle sensory neurons. Development 129:4879–4889
Gomez-Smith M, Qin Z, Zhou X, Schock SC, Chen HH (2010) LIM domain only 4 protein promotes granulocyte colony-stimulating factor-induced signaling in neurons. Cell Mol Life Sci 67:949–957
Novotny-Diermayr V, Lin B, Gu L, Cao X (2005) Modulation of the interleukin-6 receptor subunit glycoprotein 130 complex and its signaling by LMO4 interaction. J Biol Chem 280:12747–12757
Schock SC, Xu J, Duquette PM, Qin Z, Rai PS, Lewandowski AJ, Thompson CS, Seifert EL, Harper ME, Chen HH (2008) Rescue of neurons from ischemic injury by PPAR-gamma requires a novel essential cofactor LMO4. J Neurosci 28:12433–12444
Chen HH, Xu J, Safarpour F, Stewart AF (2007) LMO4 mRNA stability is regulated by extracellular ATP in F11 cells. Biochem Biophys Res Commun 357:56–61
Kenny DA, Jurata LW, Saga Y, Gill GN (1998) Identification and characterization of LMO4, an LMO gene with a novel pattern of expression during embryogenesis. Proc Natl Acad Sci USA 95:11257–11262
Hahm K, Sum EY, Fujiwara Y, Lindeman GJ, Visvader JE, Orkin SH (2004) Defective neural tube closure and anteroposterior patterning in mice lacking the LIM protein LMO4 or its interacting partner Deaf-1. Mol Cell Biol 24:2074–2082
Ziegler DR, Cullinan WE, Herman JP (2002) Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol 448:217–229
Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG (2006) Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494:673–685
Li DP, Chen SR, Pan HL (2004) VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol 92:1807–1816
Ward KR, Bardgett JF, Wolfgang L, Stocker SD (2011) Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57:435–441
Shi H, Bowers RR, Bartness TJ (2004) Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav 81:535–542
Duquette PM, Zhou X, Yap NL, Maclaren EJ, Lu JJ, Wallace VA, Chen HH (2010) Loss of LMO4 in the retina leads to reduction of GABAergic amacrine cells and functional deficits. PLoS One 5:e13232
Kaiyala KJ, Schwartz MW (2011) Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60:17–23
Sell H, Berger JP, Samson P, Castriota G, Lalonde J, Deshaies Y, Richard D (2004) Peroxisome proliferator-activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145:3925–3934
Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB (2006) Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol 291:R1613–R1621
Myers MG Jr, Munzberg H, Leinninger GM, Leshan RL (2009) The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9:117–123
Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U (2003) The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431
Choi S, Sparks R, Clay M, Dallman MF (1999) Rats with hypothalamic obesity are insensitive to central leptin injections. Endocrinology 140:4426–4433
Minokoshi Y, Saito M, Shimazu T (1986) Sympathetic denervation impairs responses of brown adipose tissue to VMH stimulation. Am J Physiol 251:R1005–R1008
Vander Tuig JG, Knehans AW, Romsos DR (1982) Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sci 30:913–920
Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL (2008) Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149:2138–2148
Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB (2005) Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71
Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ (1998) Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol 275:R291–R299
Duplan SM, Boucher F, Alexandrov L, Michaud JL (2009) Impact of Sim1 gene dosage on the development of the paraventricular and supraoptic nuclei of the hypothalamus. Eur J Neurosci 30:2239–2249
Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR (2008) Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 22:1723–1734
Zoeller RT, Kabeer N, Albers HE (1990) Cold exposure elevates cellular levels of messenger ribonucleic acid encoding thyrotropin-releasing hormone in paraventricular nucleus despite elevated levels of thyroid hormones. Endocrinology 127:2955–2962
McMinn JE, Liu SM, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, Chua SC Jr (2005) Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab 289:E403–E411
Zhang EE, Chapeau E, Hagihara K, Feng GS (2004) Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA 101:16064–16069
El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS (2000) Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105:1827–1832
Buckland PR, Hoogendoorn B, Guy CA, Coleman SL, Smith SK, Buxbaum JD, Haroutunian V, O’Donovan MC (2004) A high proportion of polymorphisms in the promoters of brain expressed genes influences transcriptional activity. Biochim Biophys Acta 1690:238–249
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307–320
Mutsuga N, Shahar T, Verbalis JG, Xiang CC, Brownstein MJ, Gainer H (2005) Regulation of gene expression in magnocellular neurons in rat supraoptic nucleus during sustained hypoosmolality. Endocrinology 146:1254–1267
Cirelli C, Tononi G (2000) Gene expression in the brain across the sleep-waking cycle. Brain Res 885:303–321
Acknowledgments
This work is supported by grants from the Canadian Diabetes Association, Ontario Research Fund, Canada Foundation for Innovation, Heart and Stroke Foundation of Canada (Gant-in-Aid NA6301), and Canadian Institutes of Health Research (MOP-179197) to H.–H. Chen. H.–H. Chen is also supported by the Henry J. M. Barnett Research Scholarship and New Investigator Award from the HSFC and by an Early Researcher Award from the Ontario Ministry of Research and Innovation. Z. Qin has been supported by scholarships from the Ontario Graduate Program, the Natural Science and Engineering Research Council and University of Ottawa. We thank Dr. Diane Lagace and the behavior core facility at the University of Ottawa for assistance on locomotor activity measurements by autonomic beam break. We are grateful for Drs. Alexander Sorisky (Ottawa Hospital Research Institute) and Alexandre F.R. Stewart (University of Ottawa Heart Institute) for helpful discussions and reading the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zhou, X., Gomez-Smith, M., Qin, Z. et al. Ablation of LMO4 in glutamatergic neurons impairs leptin control of fat metabolism. Cell. Mol. Life Sci. 69, 819–828 (2012). https://doi.org/10.1007/s00018-011-0794-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0794-3