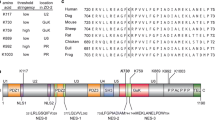
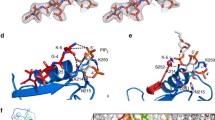
Abstract
Zonula occludens proteins (ZO) are postsynaptic density protein-95 discs large-zonula occludens (PDZ) domain-containing proteins that play a fundamental role in the assembly of tight junctions and establishment of cell polarity. Here, we show that the second PDZ domain of ZO-1 and ZO-2 binds phosphoinositides (PtdInsP) and we identified critical residues involved in the interaction. Furthermore, peptide and PtdInsP binding of ZO PDZ2 domains are mutually exclusive. Although lipid binding does not seem to be required for plasma membrane localisation of ZO-1, phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P 2) binding to the PDZ2 domain of ZO-2 regulates ZO-2 recruitment to nuclear speckles. Knockdown of ZO-2 expression disrupts speckle morphology, indicating that ZO-2 might play an active role in formation and stabilisation of these subnuclear structures. This study shows for the first time that ZO isoforms bind PtdInsPs and offers an alternative regulatory mechanism for the formation and stabilisation of protein complexes in the nucleus.







Similar content being viewed by others
References
Cho W (2006) Building signaling complexes at the membrane. Sci STKE, e7
Overduin M, Cheever ML, Kutateladze TG (2001) Signaling with phosphoinositides: better than binary. Mol Interv 1:150–159
Hammond GR, Schiavo G (2007) Polyphosphoinositol lipids: under-PPInning synaptic function in health and disease. Dev Neurobiol 67:1232–1247
Wymann MP, Schneiter R (2008) Lipid signalling in disease. Nat Rev Mol Cell Biol 9:162–176
Hammond G, Thomas CL, Schiavo G (2004) Nuclear phosphoinositides and their functions. Curr Top Microbiol Immunol 282:177–206
Payrastre B, Missy K, Giuriato S, Bodin S, Plantavid M, Gratacap M (2001) Phosphoinositides: key players in cell signalling, in time and space. Cell Signal 13:377–387
Toker A (2002) Phosphoinositides and signal transduction. Cell Mol Life Sci 59:761–779
Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K (2006) Phosphatidylinositol-3, 4, 5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8:963–970
Martin-Belmonte F, Mostov K (2008) Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol 20:227–234
Matter K, Balda MS (2007) Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci 120:1505–1511
Aijaz S, Balda MS, Matter K (2006) Tight junctions: molecular architecture and function. Int Rev Cytol 248:261–298
Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286:C1213–C1228
Ebnet K (2008) Organization of multiprotein complexes at cell–cell junctions. Histochem Cell Biol 130:1–20
Islas S, Vega J, Ponce L, Gonzalez-Mariscal L (2002) Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp Cell Res 274:138–148
Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H (2003) The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem 278:2692–2700
Gottardi CJ, Arpin M, Fanning AS, Louvard D (1996) The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell–cell contacts. Proc Natl Acad Sci USA 93:10779–10784
Gonzalez-Mariscal L, Ponce A, Alarcon L, Jaramillo BE (2006) The tight junction protein ZO-2 has several functional nuclear export signals. Exp Cell Res 312:3323–3335
Jaramillo BE, Ponce A, Moreno J, Betanzos A, Huerta M, Lopez-Bayghen E, Gonzalez-Mariscal L (2004) Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells. Exp Cell Res 297:247–258
Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L (2004) The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res 292:51–66
Kavanagh E, Buchert M, Tsapara A, Choquet A, Balda MS, Hollande F, Matter K (2006) Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci 119:5098–5105
Huang HY, Li R, Sun Q, Wang J, Zhou P, Han H, Zhang WH (2002) LIM protein KyoT2 interacts with human tight junction protein ZO-2-i3. Yi Chuan Xue Bao 29:953–958
Huerta M, Munoz R, Tapia R, Soto-Reyes E, Ramirez L, Recillas-Targa F, Gonzalez-Mariscal L, Lopez-Bayghen E (2007) Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc. Mol Biol Cell 18:4826–4836
Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M (2007) PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28:886–898
Zimmermann P, Meerschaert K, Reekmans G, Leenaerts I, Small JV, Vandekerckhove J, David G, Gettemans J (2002) PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol Cell 9:1215–1225
Kates M (1986) Techniques of lipidology, 2nd edn. Elsevier, Amsterdam
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Hammond GR, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G (2006) Elimination of plasma membrane phosphatidylinositol (4, 5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci 119:2084–2094
Hammond GR, Schiavo G, Irvine RF (2009) Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P 2. Biochem J (in press)
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Manna D, Albanese A, Park WS, Cho W (2007) Mechanistic basis of differential cellular responses of phosphatidylinositol 3, 4-bisphosphate- and phosphatidylinositol 3, 4, 5-trisphosphate-binding pleckstrin homology domains. J Biol Chem 282:32093–32105
Rusten TE, Stenmark H (2006) Analyzing phosphoinositides and their interacting proteins. Nat Methods 3:251–258
Varnai P, Balla T (1998) Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol 143:501–510
Fanning AS, Lye MF, Anderson JM, Lavie A (2007) Domain swapping within PDZ2 is responsible for dimerization of ZO proteins. J Biol Chem 282:37710–37716
Giepmans BN, Verlaan I, Moolenaar WH (2001) Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun Adhes 8:219–223
Chen J, Pan L, Wei Z, Zhao Y, Zhang M (2008) Domain-swapped dimerization of ZO-1 PDZ2 generates specific and regulatory connexin43-binding sites. EMBO J 27:2113–2123
Flores CE, Li X, Bennett MV, Nagy JI, Pereda AE (2008) Interaction between connexin35 and zonula occludens-1 and its potential role in the regulation of electrical synapses. Proc Natl Acad Sci USA 105:12545–12550
Nourry C, Grant SG, Borg JP (2003) PDZ domain proteins: plug and play! Sci STKE, RE7
Mortier E, Wuytens G, Leenaerts I, Hannes F, Heung MY, Degeest G, David G, Zimmermann P (2005) Nuclear speckles and nucleoli targeting by PIP2-PDZ domain interactions. EMBO J 24:2556–2565
Osborne SL, Thomas CL, Gschmeissner S, Schiavo G (2001) Nuclear PtdIns(4, 5)P 2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci 114:2501–2511
Hernandez S, Chavez MB, Gonzalez-Mariscal L (2007) ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp Cell Res 313:1533–1547
Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W (2008) Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol 28:1669–1678
Kachel N, Erdmann KS, Kremer W, Wolff P, Gronwald W, Heumann R, Kalbitzer HR (2003) Structure determination and ligand interactions of the PDZ2b domain of PTP-Bas (hPTP1E): splicing-induced modulation of ligand specificity. J Mol Biol 334:143–155
Cho W, Stahelin RV (2005) Membrane–protein interactions in cell signaling and membrane trafficking. Annu Rev Biophys Biomol Struct 34:119–151
Bunce MW, Bergendahl K, Anderson RA (2006) Nuclear PI(4, 5)P(2): a new place for an old signal. Biochim Biophys Acta 1761:560–569
Reichert M, Muller T, Hunziker W (2000) The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin–Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem 275:9492–9500
Fanning AS, Little BP, Rahner C, Utepbergenov D, Walther Z, Anderson JM (2007) The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell 18:721–731
van Zeijl L, Ponsioen B, Giepmans BN, Ariaens A, Postma FR, Varnai P, Balla T, Divecha N, Jalink K, Moolenaar WH (2007) Regulation of connexin43 gap junctional communication by phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 177:881–891
Tapia R, Huerta M, Islas S, vila-Flores A, Lopez-Bayghen E, Weiske J, Huber O, Gonzalez-Mariscal L (2009) Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle. Mol Biol Cell 20:1102–1117
Harris BZ, Lim WA (2001) Mechanism and role of PDZ domains in signaling complex assembly. J Cell Sci 114:3219–3231
Acknowledgments
We thank Dr. E. Mortier for helpful discussions during the course of this project and Dr. L. Van Troys and Dr. G. Hammond for useful advice on the lipid stainings. This work was supported by the Fund for Scientific Research-Flanders (FWO-Vlaanderen), the Concerted Actions Programme of Ghent University (GOA), the Interuniversity attraction poles (IUAP06), the Human Frontier Science Program (HFSP), a NIH grant (GM68849) (for W.C.), and the Catalyst Award from Chicago Biomedical Consortium (for W.C. and H.L.). K.M. was supported by a Postdoctoral Fellowship of the Fund for Scientific Research-Flanders (Belgium) (FWO-Vlaanderen). E.R. is supported by a fellowship from the research council of Ghent University (BOF).
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Meerschaert, M. P. Tun and E. Remue contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2009_156_MOESM1_ESM.pdf
Supplementary Figure S1. ELISA PtdInsP binding assay with PDZ domains 1-3 of ZO-2. Each well of a ‘PIP specificity’ microtiter plate (Echelon Biosciences) was overlaid with GST, GST- PH-PLC-d1 (positive control) and GST-ZO2 PDZ1-3 at a final concentration of 10 nM. Data represent means ± STDEV (n = 3). A.U. = absorbance unit (PDF 39 kb)
18_2009_156_MOESM2_ESM.pdf
Supplementary Figure S2. Gel filtration chromatography of GST-ZO1-PDZ2 (red line). Molecular weight standards (blue line) include blue dextran, immunoglobin G (IgG; 150 kDa), bovine serum albumin (BSA; 67 kDa) and ovalbumin (43 kDa). 100 nM to 5 µM GST-ZO1-PDZ2 (and GST-ZO2-PDZ2) showed the same elution pattern. The estimated molecular weight of the protein was 78 kDa, which approximates that of the GST-ZO1-PDZ2 dimer (PDF 42 kb)
18_2009_156_MOESM3_ESM.pdf
Supplementary material 3Supplementary Figure S3. A) PtdInsP selectivity of ZO-2 PDZ2 determined by kinetic SPR measurements. 1 µM of protein was added to POPC/POPE/POPS/PtdInsP (37:40:20:3) vesicles containing 7 different PtdInsPs. (B) Effects of mutations of basic residues on binding of the ZO-2 PDZ2 to POPC/POPE/POPS/PtdIns(4,5)P 2 (37:40:20:3) vesicles measured by kinetic SPR analysis. Protein concentrations were kept at 1 µM. Kd values were determined by equilibrium SPR analysis as shown in Figure 2B and 2C and listed in Table II. (PDF 79 kb)
18_2009_156_MOESM4_ESM.pdf
Supplementary Figure S4. Expression of wild-type and mutant ZO constructs in MDCK cells. (A) Expression of various GFP-tagged ZO-1 mutants defective in lipid and/or peptide binding does not affect plasma membrane localisation of ZO-1. (B) Similar experiments for ZO-2. WT=wild type. Scale bar = 10µm. (PDF 641 kb)
Rights and permissions
About this article
Cite this article
Meerschaert, K., Tun, M.P., Remue, E. et al. The PDZ2 domain of zonula occludens-1 and -2 is a phosphoinositide binding domain. Cell. Mol. Life Sci. 66, 3951–3966 (2009). https://doi.org/10.1007/s00018-009-0156-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-009-0156-6