Abstract
Background
The availability of robust biomarkers of endothelial activation might enhance the identification of subclinical atherosclerosis in rheumatoid arthritis (RA). We investigated this issue by conducting a systematic review and meta-analysis of cell adhesion molecules in RA patients.
Methods
We searched electronic databases from inception to 31 July 2023 for case–control studies assessing the circulating concentrations of immunoglobulin-like adhesion molecules (vascular cell, VCAM-1, intercellular, ICAM-1, and platelet endothelial cell, PECAM-1, adhesion molecule-1) and selectins (E, L, and P selectin) in RA patients and healthy controls. Risk of bias and certainty of evidence were assessed using the JBI checklist and GRADE, respectively.
Results
In 39 studies, compared to controls, RA patients had significantly higher concentrations of ICAM-1 (standard mean difference, SMD = 0.81, 95% CI 0.62–1.00, p < 0.001; I2 = 83.0%, p < 0.001), VCAM-1 (SMD = 1.17, 95% CI 0.73–1.61, p < 0.001; I2 = 95.8%, p < 0.001), PECAM-1 (SMD = 0.82, 95% CI 0.57–1.08, p < 0.001; I2 = 0.0%, p = 0.90), E-selectin (SMD = 0.64, 95% CI 0.42–0.86, p < 0.001; I2 = 75.0%, p < 0.001), and P-selectin (SMD = 1.06, 95% CI 0.50–1.60, p < 0.001; I2 = 84.8%, p < 0.001), but not L-selectin. In meta-regression and subgroup analysis, significant associations were observed between the effect size and use of glucocorticoids (ICAM-1), erythrocyte sedimentation rate (VCAM-1), study continent (VCAM-1, E-selectin, and P-selectin), and matrix assessed (P-selectin).
Conclusions
The results of our study support a significant role of cell adhesion molecules in mediating the interplay between RA and atherosclerosis. Further studies are warranted to determine whether the routine use of these biomarkers can facilitate the detection and management of early atherosclerosis in this patient group.
PROSPERO Registration Number: CRD42023466662.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite significant advances, particularly over the last two decades, in diagnosis, treatment, and follow-up, patients with rheumatoid arthritis (RA) continue to experience poor quality of life and inadequate social engagement [1,2,3,4,5]. There is also increasing evidence that the coexistence of RA and specific comorbidities exerts an additional public health and financial burden on patients and the healthcare workforce [6,7,8]. In particular, the link between RA and atherosclerosis is well established given the high prevalence of cardiovascular risk factors [9,10,11,12,13,14], the high incidence of myocardial infarction and stroke [15,16,17,18,19], and the recognition that cardiovascular disease represents the leading cause of mortality in RA patients [20, 21]. To further corroborate the link between RA and atherosclerosis, a considerable body of research has demonstrated significant alterations in the endothelial synthesis of the critical endogenous messenger, nitric oxide [22, 23], impaired endothelial and flow-mediated vasodilatation [24,25,26,27], intima–media thickening [28], arterial stiffening [29], and increased risk of hypertension in RA patients [30]. These abnormalities are often observed in the early phases of atherosclerosis, reflecting a state of endothelial activation and early damage of the tunica intima of the arterial wall [31, 32], and therefore their detection might instigate timely prevention strategies [33,34,35].
At a cellular and molecular level, the early stages of atherosclerosis are characterized by the adhesion of leukocytes and lymphocytes to the endothelium. This, in turn, facilitates the migration of these cells to the tunica intima [36, 37]. The process of cellular adhesion to the endothelium is mediated by a number of molecules, including the immunoglobulin-like vascular cell adhesion molecule-1 (VCAM-1), the intercellular vascular adhesion molecule-1 (ICAM-1), and the platelet endothelial cell adhesion molecule-1 (PECAM-1) [38,39,40,41]. VCAM-1 is primarily expressed in endothelial cells and macrophages and its binding to integrin α4β1 mediates its biological effects [42, 43]. ICAM-1 is upregulated in the presence of excess inflammation and binds to the leukocyte specific β2 integrins [44, 45]. PECAM-1 is expressed in leukocytes, platelets, and endothelial cells, and favors the migration of leukocytes through the translocation of the integrin α6β1 [46]. The immunoglobulin-like vascular cell adhesion molecules can be measured in plasma or serum [41, 47, 48], and their concentrations have been shown to be positively associated with cardiovascular risk [49,50,51,52,53]. Another group of molecules facilitating cell adhesion to the endothelium includes the selectins, specifically P-selectin, expressed in platelets and endothelial cells, L-selectin, expressed in leukocytes, and E-selectin, expressed in endothelial cells [54,55,56]. Selectins mediate the rolling of monocytes, neutrophils, and lymphocytes [57, 58], can also be measured in plasma or serum, and have been shown to play an important pathophysiological role in RA, angiogenesis, and atherosclerosis [59,60,61,62,63,64,65,66,67,68].
Therefore, given the robust association between RA and atherosclerosis and the potential utility of cell adhesion molecules in the identification of early, subclinical atherosclerosis, we conducted a systematic review and meta-analysis of the circulating concentrations of VCAM-1, ICAM-1, PECAM-1, P-selectin, L-selectin, and E-selectin in RA patients and healthy controls. We hypothesized that RA is associated with a significant upregulation of cell adhesion molecules, suggesting endothelial activation and dysfunction in this patient group.
Methods
Search strategy and study selection
We searched PubMed, Scopus, and Web of Science from inception to 31 July 2023 for relevant articles using the following terms and their combinations: “rheumatoid arthritis” AND “soluble cell adhesion molecules” OR “intercellular adhesion molecule-1” OR “ICAM-1” OR “sICAM-1” OR “ICAM” OR “vascular cell adhesion molecule-1” OR “VCAM-1” OR “sVCAM-1” OR “VCAM” OR “platelet endothelial cell adhesion molecule-1” OR “PECAM-1” OR “sPECAM-1” OR “PECAM” OR “selectin” or “P-selectin” OR “sP-selectin” OR “L-selectin” OR “sL-selectin” OR “E-selectin” OR “sE-selectin”. Two investigators independently screened the abstracts and, if relevant, the full articles, including their references, according to the following criteria: (a) assessment of soluble ICAM-1, VCAM-1, PECAM-1, E-selectin, L-selectin, and P-selectin in plasma or serum, (b) comparison of RA patients and healthy controls in a case–control study, (c) age of participants ≥ 18 years, (d) publications in English language, and (e) full-text article available.
The following information was independently extracted from selected manuscripts: year of publication, first author, study country and continent, number of RA patients and controls, age, sex distribution, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), RA duration, disease activity score-28 (DAS-28), matrix used for assessment (serum or plasma), method used to measure adhesion molecules, and use of methotrexate, glucocorticoids, and disease-modifying antirheumatic drugs (DMARDs).
The risk of bias was assessed using the Joanna Briggs Institute Critical Appraisal Checklist for analytical studies and the certainty of evidence using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group system [69, 70]. We complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (Supplementary Tables S1 and S2) [71]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO Registration No. CRD42023466662).
Statistical analysis
We calculated standardized mean differences (SMDs) and 95% confidence intervals (CIs) to generate forest plots of continuous data and investigate differences in the concentrations of cell adhesion molecules between RA patients and healthy controls (a p value < 0.05 was considered statistically significant) [72,73,74]. The heterogeneity of SMD across studies was evaluated using the Q-statistic (a p value p < 0.10 was considered statistically significant). Heterogeneity was considered low when I2 ≤ 25%, moderate when 25% < I2 < 75%, and high when I2 ≥ 75%. A random-effects model based on the inverse-variance method was used in case of high heterogeneity [75, 76]. The stability of the results was assessed using sensitivity analysis [77]. The Begg’s and Egger’s tests (a p-value < 0.05 was considered statistically significant) and the “trim-and-fill” method were used to assess publication bias [78,79,80]. Univariate meta-regression and subgroup analyses were performed to investigate associations between the effect size and the following parameters: year of publication, geographical area where the study was conducted, sample size, age, sex distribution, CRP, ESR, RA duration, DAS-28, sample matrix (serum or plasma), analytical method, and the use of methotrexate, glucocorticoids, and DMARDs. All statistical analyses were performed using Stata 14 (Stata Corp., College Station, TX, USA).
Results
Study selection
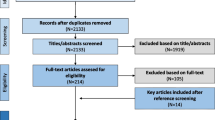
Our search identified 2055 articles. Of them, 2004 were excluded following the initial screening as they were either duplicates or not relevant. A full-text review of the remaining 51 articles led to the further exclusion of 5 studies because of duplicate data, 4 because they were not case–control, 2 because they had missing data, and 1 because it was not written in English. Therefore, 39 studies were selected for analysis (Fig. 1 and Table 1) [72, 81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118]. The initial level of certainty was rated as low (rating 2) given the cross-sectional design of all studies.
ICAM-1
Twenty-four studies investigated ICAM-1 in a total of 1857 RA patients (mean age 52 years, 84% females) and 1476 healthy controls (mean age 48 years, 81% females) [81,82,83,84,85,86, 88, 89, 91, 92, 94, 97, 100, 101, 103,104,105, 107, 109, 111, 114,115,116, 118]. Eighteen studies were conducted in Europe [82,83,84,85,86, 88, 89, 91, 94, 100, 103, 104, 109, 111, 114,115,116, 118], 4 in America [81, 101, 105, 107], 1 in Asia [92], and 1 in Africa [97]. ICAM-1 was measured using an enzyme-linked immunosorbent assay (ELISA) in all but one study, which did not provide relevant details regarding the analytical method used [100]. Eighteen studies measured ICAM-1 in serum [81,82,83,84,85,86, 88, 89, 91, 92, 94, 105, 107, 109, 111, 114, 116, 118], 2 in plasma [104, 115], while the remaining 4 did not provide relevant information [97, 101, 103, 113]. RA duration was reported in 18 studies and ranged between 0.25 and 23 years [81, 82, 84, 85, 88, 89, 92, 94, 97, 100, 101, 103, 105, 109, 111, 114, 115, 118], whereas DAS-28, reported in six studies, ranged between 2.8 and 6.23 [103, 105, 109, 114, 116, 118]. Eleven studies reported data on methotrexate treatment [85, 86, 88, 91, 92, 94, 104, 111, 114, 116, 118], 18 on glucocorticoid treatment [81, 84, 86, 88, 91, 92, 94, 97, 100, 101, 103,104,105, 109, 114, 116, 118], and 14 on DMARDs treatment [81, 84, 86, 88, 89, 91, 94, 97, 100, 103, 105, 109, 116, 118]. The risk of bias was low in 11 studies [89, 97, 100, 101, 104, 105, 107, 109, 111, 114, 116], moderate in 12 [81,82,83,84,85,86, 88, 91, 92, 94, 115, 118], and high in the remaining 1 [103] (Table 2).
The forest plot showed that RA patients had significantly higher ICAM-1 concentrations when compared to healthy controls (SMD = 0.81, 95% CI 0.62–1.00, p < 0.001; I2 = 83.0%, p < 0.001; Fig. 2). The results were stable in sensitivity analysis, with pooled SMD values ranging between 0.68 and 0.84 (Supplementary Fig. S1). There was significant publication bias according to the Begg’s test (p = 0.003) and the Egger’s test (p = 0.007). The “trim-and-fill” method identified six missing studies to be added to the left side of the funnel plot to ensure symmetry (Supplementary Fig. S2). The resulting effect size remained significant (SMD = 0.60, 95% CI 0.38–0.81, p < 0.001).
In meta-regression, there were non-significant associations between the effect size and age (t = 0.50, p = 0.62), sex distribution (t = − 0.81, p = 0.43), publication year (t = − 0.81, p = 0.43), number of participants (t = − 1.29, p = 0.21), CRP (t = 1.41, p = 0.19), ESR (t = 1.08, p = 0.31), RA duration (t = − 1.20, p = 0.25), or use of methotrexate (t = − 0.96, p = 0.36), and DMARDs (t = − 0.49, p = 0.63). By contrast, there was a significant inverse association between the effect size and the use of glucocorticoids (t = − 2.44, p = 0.027) (Fig. 3a, b). In subgroup analysis, there were non-significant differences (p = 0.70) in pooled SMD between European (SMD = 0.72, 95% CI 0.56–0.88, p < 0.001; I2 = 67.6%, p < 0.001) and American studies (SMD = 0.62, 95% CI 0.46–0.79, p < 0.001; I2 = 0.0%, p = 0.69; Supplementary Fig. S3) with a virtually absent heterogeneity in the American subgroup. Similarly, there were non-signficant differences (p = 0.83) in pooled SMD between studies measuring serum (SMD = 0.93, 95% CI 0.67–1.18, p < 0.001; I2 = 86.4%, p < 0.001) and plasma (SMD = 0.86, 95% CI 0.62–1.11, p < 0.001; I2 = 0.0%, p = 0.58; Supplementary Fig. S4), with a virtually absent heterogeneity in the plasma subgroup.
The overall level of certainty was upgraded to moderate (rating 3) after considering the low-moderate risk of bias in most studies (no change), the high but partly explainable heterogeneity (no change), the lack of indirectness (no change), the relatively large effect size (SMD = 0.81, upgrade one level) [119], and the presence of publication bias which was addressed with the “trim-and-fill” method (no change).
VCAM-1
Twenty studies (21 comparator groups) investigated VCAM-1 in a total of 1446 RA patients (mean age 50 years, 81% females) and 1044 healthy controls (mean age 48 years, 79% females) [72, 83, 86, 88, 93, 97, 98, 100, 103,104,105,106,107, 109,110,111,112, 116,117,118]. Fourteen studies were conducted in Europe [83, 86, 88, 98, 100, 103, 104, 106, 109,110,111,112, 116, 118], 3 in Africa [93, 97, 117], 2 in America [105, 107], and 1 in Asia [72]. VCAM-1 was measured using an ELISA assay in all but one study for all studies except for one in which authors did not declare the method employed [100]. Fourteen studies investigated ICAM-1 in serum [72, 83, 86, 88, 93, 98, 105, 107, 109,110,111, 116,117,118] and 2 in plasma [104, 112]. No relevant information regarding the matrix analysed was provided in the remaining four studies [97, 100, 103, 106]. RA duration was reported in 12 studies and ranged between 0.25 and 23 years [88, 93, 97, 98, 100, 103, 105, 109,110,111,112, 118], whereas the DAS-28 was reported in 11 study comparators and ranged between 2.3 and 6.23 [98, 103, 105, 106, 109, 110, 112, 116,117,118]. The use of methotrexate was reported in 10 studies [72, 86, 88, 98, 104, 110, 111, 116,117,118], glucocorticoids in 14 [72, 86, 88, 97, 100, 103,104,105,106, 109, 112, 116,117,118], and DMARDs in 13 [72, 86, 88, 93, 97, 100, 103, 105, 106, 109, 110, 116, 117]. The risk of bias was low in 13 studies [93, 97, 98, 100, 104, 105, 107, 109,110,111,112, 116, 117], moderate in 4 [83, 86, 88, 118], and high in the remaining 3 [72, 103, 106] (Table 2).
The forest plot showed that VCAM-1 concentrations were significantly higher in RA patients when compared to controls (SMD = 1.17, 95% CI 0.73–1.61, p < 0.001; I2 = 95.8%, p < 0.001; Fig. 4). The pooled SMD values were stable in sensitivity analysis, ranging between 0.94 and 1.27 (Supplementary Fig. S5). There was a significant publication bias (Begg’s test, p = 0.006, and Egger’s test, p = 0.009). The “trim-and-fill” method identified eight missing studies to be added to the left side of the funnel plot to ensure symmetry (Supplementary Fig. S6). However, the resulting effect size was no longer significant (SMD = 0.39, 95% CI − 0.11 to 0.88, p = 0.12).
Non-significant associations were observed in the meta-regression between the effect size and age (t = − 0.86, p = 0.40), sex distribution (t = 1.38, p = 0.19), year of publication (t = 0.78, p = 0.45), sample size (t = − 0.33, p = 0.74), CRP (t = 1.03, p = 0.33), RA duration (t = − 0.10, p = 0.92), or use of methotrexate (t = − 1.62, p = 0.14) and glucocorticoids (t = − 1.05, p = 0.32). However, there was a trend toward a significant association between the effect size and DMARDs use (t = − 2.00, p = 0.07) and a significant association between SMD and ESR (t = 2.30, p = 0.047; Fig. 5a, b). In subgroup analysis, there was a significant difference (p < 0.001) in pooled SMD between American (SMD = 0.26, 95% CI − 0.20 to 0.71, p = 0.27; I2 = 76.2%, p = 0.041), European (SMD = 0.80, 95% CI 0.36–1.24, p < 0.001; I2 = 93.2%, p < 0.001), and African studies (SMD = 1.77, 95% CI 1.21–2.33, p < 0.001; I2 = 82.7%, p < 0.001; Fig. 6). By contrast, there were non-significant differences (p = 0.63) in pooled SMD between studies investigating serum (SMD = 1.18, 95% CI 0.63–1.73, p < 0.001; I2 = 96.3%, %, p < 0.001) and plasma (SMD = 0.61, 95% CI 0.29–0.92, p = 0.22; I2 = 0.0%, p = 0.455; Supplementary Fig. S7), with a virtual absence of heterogeneity in the plasma subgroup.
The overall level of certainty remained low (rating 2) after considering the low–moderate risk of bias in most studies (no change), the high but partly explainable heterogeneity (no change), the lack of indirectness (no change), the relatively large effect size (SMD = 1.17, upgrade one level) [119], and the presence of publication bias which was not fully addressed with the “trim-and-fill” method (downgrade one level).
PECAM-1
Two studies, one conducted in Europe [103], and the other in Asia [113], investigated PECAM-1 in a total of 130 RA patients (mean age 55 years, 80% females) and 124 healthy controls (mean age 43 years, 81% females). PECAM-1 was measured using an ELISA in both studies, in serum in one study [113], whereas the second study did not provide any details regarding the matrix assessed [103]. The risk of bias was low in one study [113] and high in the other [103] (Table 2). The forest plot showed that RA patients had significantly higher PECAM-1 concentrations when compared to controls (SMD = 0.82, 95% CI 0.57–1.08, p < 0.001; I2 = 0.0%, p = 0.905; Fig. 7). Sensitivity analysis, assessment of publication bias, and meta-regression and subgroup analysis could not be conducted because of the limited number of studies.
The overall level of certainty remained low (rating 2) after considering the low risk of bias in one of the two studies (no change), the virtually absent heterogeneity (no change), the lack of indirectness (no change), the relatively large effect size (SMD = 0.82, upgrade one level) [119], and the lack of assessment of publication bias (downgrade one level).
E-selectin
Eighteen studies investigated E-selectin in a total of 831 RA patients (mean age 53 years, 78% females) and 720 healthy controls (mean age 50 years, 72% females) [83, 84, 86, 88, 89, 91, 93, 95, 96, 99, 102,103,104, 106,107,108,109, 112]. Twelve studies were conducted in Europe [83, 84, 86, 88, 89, 91, 103, 104, 106, 108, 109, 112], 2 in America [99, 107], 2 in Asia [95, 102], and 2 in Africa [93, 96]. E-selectin was measured using an ELISA in all studies. Measurements were performed in serum in 13 studies [83, 84, 86, 88, 89, 91, 93, 95, 96, 99, 107,108,109] and plasma in 3 [102, 104, 112]. Details regarding the matrix assessed were missing in two studies [103, 106]. RA duration, reported in 12 studies, ranged between 0.25 and 23 years [84, 88, 89, 93, 95, 96, 99, 102, 103, 108, 109, 112], whereas the DAS-28 reported in 6 studies ranged between 3.6 and 6.23 [99, 103, 106, 108, 109, 112]. Twelve studies reported data on glucocorticoid treatment [84, 86, 88, 91, 99, 102,103,104, 106, 108, 109, 112] and 12 on DMARDs treatment [84, 86, 88, 89, 91, 93, 99, 102, 103, 106, 108, 109]. The risk of bias was low in seven studies [89, 93, 99, 104, 107, 109, 112], moderate in eight [83, 84, 86, 88, 91, 95, 96, 102], and high in the remaining three [103, 106, 108].
The forest plot showed that E-selectin concentrations were significantly higher in RA patients when compared to controls (SMD = 0.64, 95% CI 0.42–0.86, p < 0.001; I2 = 75.0%, p < 0.001; Fig. 8). Sensitivity analysis showed that the pooled SMD values were stable, ranging between 0.59 and 0.70 (Supplementary Fig. S8). There was no significant publication bias with the Begg’s test (p = 0.82) or the Egger’s test (p = 0.98). The “trim-and-fill” method identify two missing studies to be added to the left side of the funnel plot to ensure symmetry (Supplementary Fig. S9). However, the resulting effect size remained significant (SMD = 0.56, 95% CI 0.33–0.78; p < 0.001).
No significant associations were observed in meta-regression between the effect size and age (t = 0.10, p = 0.92), sex distribution (t = − 0.64, p = 0.54), publication year (t = − 1.54, p = 0.14), number of participants (t = 0.33, p = 0.75), CRP (t = 1.06, p = 0.31), ESR (t = 1.70, p = 0.12), RA duration (t = 0.83, p = 0.43), or use of DMARDs (t = − 0.54, p = 0.60) and glucocorticoids (t = − 0.87, p = 0.41). In subgroup analysis, there was a significant difference (p = 0.02) in pooled SMD between Asian (SMD = 0.43, 95% CI − 0.11 to 0.96, p = 0.12; I2 = 38.1%, p = 0.204), European (SMD = 0.51, 95% CI 0.23–0.79, p < 0.001; I2 = 76.0%, p < 0.001), American (SMD = 0.82, 95% CI 0.50–1.14, p < 0.001; I2 = 50.2%, p = 0.156), and African studies (SMD = 1.41, 95% CI 0.97–1.84, p < 0.001; I2 = 0.0%, p = 0.493; Fig. 9), with a virtually absent heterogeneity in the African subgroup. By contrast, there were non-significant differences (p = 0.61) in pooled SMD values between studies investigating serum (SMD = 0.65, 95% CI 0.36–0.95, p < 0.001; I2 = 81.4%, %, p < 0.001) and plasma (SMD = 0.51, 95% CI 0.23–0.79, p < 0.001; I2 = 0.0%, p = 0.405; Supplementary Fig. S10), with a virtually absent heterogeneity in the plasma subgroup.
The overall level of certainty remained low (rating 2) after considering the low–moderate risk of bias in most studies (no change), the moderate and partially explained heterogeneity (no change), the lack of indirectness (no change), the moderate effect size (SMD = 0.64, no change) [119], and the absence of publication bias (no change).
L-selectin
Six studies, all conducted in Europe, investigated L-selectin in a total of 230 RA patients (mean age 51 years, 80% females) and 170 healthy controls (mean age 48 years, 81% females) [86, 90, 95, 103, 108, 109]. L-selectin was measured in serum by ELISA in all studies, except one study which did not provide relevant details regarding the matrix assessed [103]. The risk of bias was low in one study [109], moderate in three [86, 90, 95], and high in the remaining two [103, 108].
The forest plot showed the absence of significant between-group differences in L-selectin concentrations (SMD = 0.21, 95% CI − 0.66 to 1.08, p = 0.63; I2 = 93.5%, p < 0.001; Fig. 10). Sensitivity analysis showed stability of the results, with pooled SMD values ranging between − 0.14 and 0.60 (Supplementary Fig. S11).
Assessment of publication bias, meta-regression and subgroup analysis could not be conducted because of the relatively small number of studies.
The overall level of certainty was downgraded to very low (rating 1) after considering the low–moderate risk of bias in most studies (no change), the high and unexplained heterogeneity (downgrade one level), the lack of indirectness (no change), the small effect size (SMD = 0.21, no change) [119], and the lack of assessment of publication bias (downgrade one level).
P-selectin
Eight studies investigated P-selectin in a total of 230 RA patients (mean age 53 years, 85% females) and 211 healthy controls (mean age 51 years, 87% females) [86,87,88, 90, 95, 103, 106, 108]. Six studies were conducted in Europe [86, 88, 90, 103, 106, 108], and two in Asia [87, 95]. P-selectin was measured using an ELISA assay in serum in four studies [86, 90, 95, 108] and in plasma in two studies [87, 88]. Two studies did not report relevant information regarding the matrix analyzed [103, 106]. The risk of bias was moderate in five studies [86,87,88, 90, 95] and high in the other three [103, 106, 108] (Table 2).
The forest plot showed that P-selectin concentrations were significantly higher in RA patients when compared to controls (SMD = 1.06, 95% CI 0.50–1.60, p < 0.001; I2 = 84.8%, p < 0.001; Fig. 11). The pooled SMD values were stable in sensitivity analysis and ranged between 0.81 and 1.21 (Supplementary Fig. S12). Assessment of publication bias and meta-regression analysis could not be performed because of the limited number of studies.
In subgroup analysis, the pooled SMD was significantly different in European studies (SMD = 0.88, 95% CI 0.39–1.38, p < 0.001; I2 = 77.6%, p < 0.001), but not in Asian studies (SMD = 1.63, 95% CI − 0.71 to 3.97, p = 0.17; I2 = 95.0%, p < 0.001; Fig. 12). A significant difference (p = 0.045) in pooled SMD was observed between studies on serum (SMD = 0.99, 95% CI 0.61–1.37, p < 0.001; I2 = 37.5%, p = 0.187), and those on plasma (SMD = 2.31, 95% CI 1.23–3.39, p < 0.001; I2 = 66.7%, p = 0.083, Fig. 13), with a decreased between-study variance in the serum subgroup.
The overall level of certainty remained low (rating 2) after considering the moderate risk of bias in most studies (no change), the high but partially explained heterogeneity (no change), the lack of indirectness (no change), the large effect size (SMD = 1.06, upgrade one level) [119], and the lack of assessment of publication bias (downgrade one level).
Discussion
Our study supports a significant pathophysiological role of cell adhesion molecules in mediating the interplay between RA and atherosclerosis. Specifically, RA patients had significantly higher concentrations of VCAM-1, ICAM-1, E-selectin, and P-selectin, but not L-selectin, when compared to healthy controls. The corresponding pooled SMD values were stable in sensitivity analysis. In meta-regression and subgroup analysis, we observed significant associations between the effect size of ICAM-1 and the use of glucocorticoids, between the effect size of VCAM-1 and ERS, between the effect size of VCAM-1, E-selectin, and P-selectin and study continent, and between the effect size of P-selectin and the type of matrix assessed (plasma vs. serum).
The atherosclerotic process is characterized by a significant dysregulation of inflammatory and immune cellular and molecular pathways [120, 121]. As part of such dysregulation, the abnormal interaction between leukocytes and endothelial cells, mediated by cell adhesion molecules, plays a critical role in the early stages of plaque formation [122]. The consequent accumulation of leukocytes in the intima layer, in turn, facilitates their uptake of lipoprotein particles and subsequent differentiation into macrophages, precursors of foam cells, critically involved in plaque growth [123,124,125]. Accordingly, several studies have reported the upregulation of immunoglobulin-like adhesion molecules and selectins in experimental models of atherosclerosis [55, 126,127,128,129].
The significant elevations in VCAM-1, ICAM-1, E-selectin, and P-selectin in RA patients observed in our systematic review and meta-analysis reflect a state of endothelial activation and dysregulation, in the context of excessive inflammation and oxidative stress. Furthermore, they suggest the potential utility of measuring cell adhesion molecules for cardiovascular risk stratification in this patient group. The lack of significant between-group differences in the concentrations of L-selectin, a critical regulator of leukocyte tethering, rolling, adhesion, migration and signaling and monocyte protrusion during trans-endothelial migration [58, 130], indicates a different pathophysiological role of this selectin in RA. However, the relatively small number of studies investigating L-selectin warrant further research to confirm this proposition. Furthermore, the lack of significant associations in meta-regression analysis between the effect size of the between-group differences in cell adhesion molecules and RA duration or DAS-28 also indicates that alterations in cell adhesion molecules are already present in patients with short disease duration and relatively low disease activity.
Another interesting observation was the significant inverse association between the effect size of ICAM-1 and the use of glucocorticoids. This finding is in line with the results of in vitro studies reporting that dexamethasone, a potent glucocorticoid, inhibits the expression of E-selectin and ICAM-1 in endothelial cells following treatment with the endotoxin lipopolysaccharide, a known stimulator of acute pro-inflammatory responses [131]. Other studies have reported a similar effect of glucocorticoids on the expression of cell adhesion molecules [132,133,134]. Accordingly, the observation of a significant positive association between the effect size of VCAM-1 and ESR support the traditional proposition that endothelial activation and dysregulation are intimately linked with excess inflammation [135,136,137]. However, as such associations were observed with specific cell adhesion molecules, i.e., ICAM-1 and VCAM-1, further research is warranted to confirm these findings in patients with RA.
In subgroup analysis, a significant association was observed the SMD of VCAM-1, E-selectin, and P-selectin and study continent, suggesting the presence of ethnic-related differences in cell adhesion molecules. Specifically, the SMD of VCAM-1 was progressively higher in American, European, and African studies, the SMD of E-selectin was progressively higher in Asian, European, American, and African studies, and the SMD of P-selectin was significant in European but not Asian studies. In an epidemiological study conducted in England, participants of African background had significantly lower concentrations of VCAM-1 and ICAM-1 when compared to Caucasian and South Asian participants [138]. Relatively higher VCAM-1 concentrations in Caucasians vs. African Americans, Hispanics, and Chinese participants have also been reported in a North American study [139]. By contrast, studies have generally failed to identify the presence of significant ethnic-related differences in circulating selectins [140,141,142,143]. It is important to emphasize however that these studies generally investigated patients with relatively low cardiovascular risk and without autoimmune diseases, suggesting that additional studies are required to investigate possible ethnic-related differences in the concentrations of cell adhesion molecules in patients with RA and other rheumatic diseases.
Strengths of our systematic review and meta-analysis include the combined assessment of a range of immunoglobulin-like adhesion molecules and selectins in patients with RA in a relatively large number of studies, and the robust assessment of the risk of bias and the certainty of evidence for each adhesion molecule. A possible limitation is related to the high heterogeneity observed for the studied adhesion molecules. However, specific sources of heterogeneity were identified for ICAM-1 (study continent and matrix assessed), VCAM-1 (matrix assessed), E-selectin (study continent and matrix assessed), and P-selectin (study continent).
In conclusion, our systematic review and meta-analysis suggests that cell adhesion molecules play an important pathophysiological role in the interplay between RA and atherosclerosis, including patients with relatively short RA duration and low disease activity. Further studies are warranted to investigate the potential utility of cell adhesion molecules in cardiovascular risk stratification and the possible effects of immunomodulatory and anti-inflammatory treatments.
Data availability
The data that support the findings of this systematic review and meta-analysis are available from AZ upon reasonable request.
References
Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78:1463–71.
Cai Y, Zhang J, Liang J, Xiao M, Zhang G, Jing Z, et al. The Burden of Rheumatoid Arthritis: Findings from the 2019 Global Burden of Diseases Study and Forecasts for 2030 by Bayesian Age-Period-Cohort Analysis. J Clin Med. 2023;12:1.
Xavier RM, Zerbini CAF, Pollak DF, Morales-Torres JLA, Chalem P, Restrepo JFM, et al. Burden of rheumatoid arthritis on patients’ work productivity and quality of life. Adv Rheumatol. 2019;59:47.
Klak A, Raciborski F, Samel-Kowalik P. Social implications of rheumatic diseases. Reumatologia. 2016;54:73–8.
Dey M, Busby A, Elwell H, Lempp H, Pratt A, Young A, et al. Association between social deprivation and disease activity in rheumatoid arthritis: a systematic literature review. RMD Open. 2022;8:1.
van Onna M, Boonen A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet Disord. 2016;17:184.
Vu M, Carvalho N, Clarke PM, Buchbinder R, Tran-Duy A. Impact of comorbid conditions on healthcare expenditure and work-related outcomes in patients with rheumatoid arthritis. J Rheumatol. 2021;48:1221–9.
Taylor PC, Atzeni F, Balsa A, Gossec L, Muller-Ladner U, Pope J. The key comorbidities in patients with rheumatoid arthritis: a narrative review. J Clin Med. 2021;10:1.
Bilecik NA, Tuna S, Samanci N, Balci N, Akbas H. Prevalence of metabolic syndrome in women with rheumatoid arthritis and effective factors. Int J Clin Exp Med. 2014;7:2258–65.
Liu XZ, Gao Y, Fan J, Xu X, Zhang J, Gao J, et al. Metabolic abnormalities in rheumatoid arthritis patients with comorbid diabetes mellitus. Clin Rheumatol. 2018;37:219–26.
Costa NT, Scavuzzi BM, Iriyoda TMV, Lozovoy MAB, Alfieri DF, de Medeiros FA, et al. Metabolic syndrome and the decreased levels of uric acid by leflunomide favor redox imbalance in patients with rheumatoid arthritis. Clin Exp Med. 2018;18:363–72.
Erre GL, Cacciapaglia F, Sakellariou G, Manfredi A, Bartoloni E, Viapiana O, et al. C-reactive protein and 10-year cardiovascular risk in rheumatoid arthritis. Eur J Intern Med. 2022;104:49–54.
Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS ONE. 2015;10:e0117952.
Cacciapaglia F, Spinelli FR, Piga M, Erre GL, Sakellariou G, Manfredi A, et al. Estimated 10-year cardiovascular risk in a large Italian cohort of rheumatoid arthritis patients: data from the Cardiovascular Obesity and Rheumatic DISease (CORDIS) Study Group. Eur J Intern Med. 2022;96:60–5.
Blum A, Adawi M. Rheumatoid arthritis (RA) and cardiovascular disease. Autoimmun Rev. 2019;18:679–90.
Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–11.
Liu W, Ma W, Liu H, Li C, Zhang Y, Liu J, et al. Stroke risk in arthritis: a systematic review and meta-analysis of cohort studies. PLoS ONE. 2021;16:e0248564.
Houri Levi E, Watad A, Whitby A, Tiosano S, Comaneshter D, Cohen AD, Amital H. Coexistence of ischemic heart disease and rheumatoid arthritis patients—a case control study. Autoimmun Rev. 2016;15:393–6.
Hansildaar R, Vedder D, Baniaamam M, Tausche AK, Gerritsen M, Nurmohamed MT. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. 2021;3:e58–70.
Reiss AB, Silverman A, Khalfan M, Vernice NA, Kasselman LJ, Carsons SE, De Leon J. Accelerated atherosclerosis in rheumatoid arthritis: mechanisms and treatment. Curr Pharm Des. 2019;25:969–86.
Figus FA, Piga M, Azzolin I, McConnell R, Iagnocco A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20:102776.
Erre GL, Mangoni AA, Castagna F, Paliogiannis P, Carru C, Passiu G, Zinellu A. Meta-analysis of asymmetric dimethylarginine concentrations in rheumatic diseases. Sci Rep. 2019;9:5426.
Mangoni AA, Tommasi S, Sotgia S, Zinellu A, Paliogiannis P, Piga M, et al. Asymmetric dimethylarginine: a key player in the pathophysiology of endothelial dysfunction, vascular inflammation and atherosclerosis in rheumatoid arthritis? Curr Pharm Des. 2021;27:2131–40.
Erre GL, Buscetta G, Paliogiannis P, Mangoni AA, Carru C, Passiu G, Zinellu A. Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta-analysis. Rheumatol Int. 2018;38:1179–90.
Erre GL, Piga M, Fedele AL, Mura S, Piras A, Cadoni ML, et al. Prevalence and determinants of peripheral microvascular endothelial dysfunction in rheumatoid arthritis patients: a multicenter cross-sectional study. Mediators Inflamm. 2018;2018:6548715.
Mangoni AA, Woodman RJ, Piga M, Cauli A, Fedele AL, Gremese E, et al. Patterns of anti-inflammatory and immunomodulating drug usage and microvascular endothelial function in rheumatoid arthritis. Front Cardiovasc Med. 2021;8:681327.
Erre GL, Mangoni AA, Passiu G, Bassu S, Castagna F, Carru C, et al. Comprehensive arginine metabolomics and peripheral vasodilatory capacity in rheumatoid arthritis: a monocentric cross-sectional study. Microvasc Res. 2020;131:104038.
Ristic GG, Lepic T, Glisic B, Stanisavljevic D, Vojvodic D, Petronijevic M, Stefanovic D. Rheumatoid arthritis is an independent risk factor for increased carotid intima-media thickness: impact of anti-inflammatory treatment. Rheumatology (Oxford). 2010;49:1076–81.
Ambrosino P, Tasso M, Lupoli R, Di Minno A, Baldassarre D, Tremoli E, Di Minno MN. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: a systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457–67.
Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, Kitas GD. Hypertension in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:1286–98.
Boulanger CM. Highlight on endothelial activation and beyond. Arterioscler Thromb Vasc Biol. 2018;38:e198–201.
Kruger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019;20:1.
Benitez RM, Vogel RA. Assessment of subclinical atherosclerosis and cardiovascular risk. Clin Cardiol. 2001;24:642–50.
Khan F, Galarraga B, Belch JJ. The role of endothelial function and its assessment in rheumatoid arthritis. Nat Rev Rheumatol. 2010;6:253–61.
Kerekes G, Soltesz P, Nurmohamed MT, Gonzalez-Gay MA, Turiel M, Vegh E, et al. Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat Rev Rheumatol. 2012;8:224–34.
Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7-12.
Jebari-Benslaiman S, Galicia-Garcia U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. 2022;23:1.
Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97.
Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203.
Zhong L, Simard MJ, Huot J. Endothelial microRNAs regulating the NF-kappaB pathway and cell adhesion molecules during inflammation. FASEB J. 2018;32:4070–84.
Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–23.
Bowden RA, Ding ZM, Donnachie EM, Petersen TK, Michael LH, Ballantyne CM, Burns AR. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res. 2002;90:562–9.
Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 2018;19:1.
Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32.
Bui TM, Wiesolek HL, Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–99.
Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med. 2002;196:1201–11.
Videm V, Albrigtsen M. Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand J Immunol. 2008;67:523–31.
Villar J, Muros M, Cabrera-Benitez NE, Valladares F, Lopez-Hernandez M, Flores C, et al. Soluble platelet-endothelial cell adhesion molecule-1, a biomarker of ventilator-induced lung injury. Crit Care. 2014;18:R41.
Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard JM, et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170:169–76.
Kaur R, Singh V, Kumari P, Singh R, Chopra H, Emran TB. Novel insights on the role of VCAM-1 and ICAM-1: potential biomarkers for cardiovascular diseases. Ann Med Surg (Lond). 2022;84:104802.
Troncoso MF, Ortiz-Quintero J, Garrido-Moreno V, Sanhueza-Olivares F, Guerrero-Moncayo A, Chiong M, et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166170.
Singh V, Kaur R, Kumari P, Pasricha C, Singh R. ICAM-1 and VCAM-1: gatekeepers in various inflammatory and cardiovascular disorders. Clin Chim Acta. 2023;548:117487.
Sahebkar A, Morris DR, Biros E, Golledge J. Association of single nucleotide polymorphisms in the gene encoding platelet endothelial cell adhesion molecule-1 with the risk of myocardial infarction: a systematic review and meta-analysis. Thromb Res. 2013;132:227–33.
Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–8.
McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–9.
Tvaroska I, Selvaraj C, Koca J. Selectins-the two Dr. Jekyll and Mr. Hyde faces of adhesion molecules—a review. Molecules. 2020;25:1.
McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–96.
Ivetic A, Hoskins Green HL, Hart SJ. L-selectin: a major regulator of leukocyte adhesion, migration and signaling. Front Immunol. 2019;10:1068.
Koch AE, Turkiewicz W, Harlow LA, Pope RM. Soluble E-selectin in arthritis. Clin Immunol Immunopathol. 1993;69:29–35.
Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–9.
Hosaka S, Shah MR, Pope RM, Koch AE. Soluble forms of P-selectin and intercellular adhesion molecule-3 in synovial fluids. Clin Immunol Immunopathol. 1996;78:276–82.
Kumar P, Hosaka S, Koch AE. Soluble E-selectin induces monocyte chemotaxis through Src family tyrosine kinases. J Biol Chem. 2001;276:21039–45.
Kumar P, Amin MA, Harlow LA, Polverini PJ, Koch AE. Src and phosphatidylinositol 3-kinase mediate soluble E-selectin-induced angiogenesis. Blood. 2003;101:3960–8.
Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–5.
Roldan V, Marin F, Lip GY, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007–20.
Bielinski SJ, Berardi C, Decker PA, Kirsch PS, Larson NB, Pankow JS, et al. P-selectin and subclinical and clinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;240:3–9.
Eikendal ALM, Bots ML, Gohar A, Lutgens E, Hoefer IE, den Ruijter HM, Leiner T. Circulating levels of P-selectin and E-selectin relate to cardiovascular magnetic resonance-derived aortic characteristics in young adults from the general population, a cross-sectional study. J Cardiovasc Magn Reson. 2018;20:54.
de Almeida-Pititto B, Ribeiro-Filho FF, Bittencourt MS, Lotufo PA, Bensenor I, Ferreira SR. Usefulness of circulating E-selectin to early detection of the atherosclerotic process in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Diabetol Metab Syndr. 2016;8:19.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors., et al., Joanna Briggs Institute reviewer’s manual. Adelaide: Johanna Briggs Institute; 2017.
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Wang L, Ding Y, Guo X, Zhao Q. Role and mechanism of vascular cell adhesion molecule-1 in the development of rheumatoid arthritis. Exp Ther Med. 2015;10:1229–33.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Shadish WR, Brasil ICC, Illingworth DA, White KD, Galindo R, Nagler ED, Rindskopf DM. Using UnGraph to extract data from image files: verification of reliability and validity. Behav Res Methods. 2009;41:177–83.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999;47:15–7.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Cush JJ, Rothlein R, Lindsley HB, Mainolfi EA, Lipsky PE. Increased levels of circulating intercellular adhesion molecule 1 in the sera of patients with rheumatoid arthritis. Arthritis Rheum. 1993;36:1098–102.
Machold KP, Kiener HP, Graninger W, Graninger WB. Soluble intercellular adhesion molecule-1 (sICAM-1) in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1993;68:74–8.
Blann AD, Herrick A, Jayson MI. Altered levels of soluble adhesion molecules in rheumatoid arthritis, vasculitis and systemic sclerosis. Br J Rheumatol. 1995;34:814–9.
Voskuyl AE, Martin S, Melchers L, Zwinderman AH, Weichselbraun I, Breedveld FC. Levels of circulating intercellular adhesion molecule-1 and -3 but not circulating endothelial leucocyte adhesion molecule are increased in patients with rheumatoid vasculitis. Br J Rheumatol. 1995;34:311–5.
Kuryliszyn-Moskal A, Bernacka K, Klimiuk PA. Circulating intercellular adhesion molecule 1 in rheumatoid arthritis–relationship to systemic vasculitis and microvascular injury in nailfold capillary microscopy. Clin Rheumatol. 1996;15:367–73.
Littler AJ, Buckley CD, Wordsworth P, Collins I, Martinson J, Simmons DL. A distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Br J Rheumatol. 1997;36:164–9.
Ertenli I, Kiraz S, Arici M, Haznedaroglu IC, Calguneri M, Celik I, Kirazli S. P-selectin as a circulating molecular marker in rheumatoid arthritis with thrombocytosis. J Rheumatol. 1998;25:1054–8.
Veale DJ, Maple C, Kirk G, McLaren M, Belch JJ. Soluble cell adhesion molecules–P-selectin and ICAM-1, and disease activity in patients receiving sulphasalazine for active rheumatoid arthritis. Scand J Rheumatol. 1998;27:296–9.
Salih AM, Nixon NB, Dawes PT, Mattey DL. Soluble adhesion molecules and anti-endothelial cell antibodies in patients with rheumatoid arthritis complicated by peripheral neuropathy. J Rheumatol. 1999;26:551–5.
Sfikakis PP, Charalambopoulos D, Vaiopoulos G, Mavrikakis M. Circulating P- and L-selectin and T-lymphocyte activation and patients with autoimmune rheumatic diseases. Clin Rheumatol. 1999;18:28–32.
Jonsson SW, Backman C, Johnson O, Karp K, Lundstrom E, Sundqvist KG, Dahlqvist SR. Increased prevalence of atherosclerosis in patients with medium term rheumatoid arthritis. J Rheumatol. 2001;28:2597–602.
Cogalgil S, Taysi S. Levels of antioxidant proteins and soluble intercellular adhesion molecule-1 in serum of patients with rheumatoid arthritis. Ann Clin Lab Sci. 2002;32:264–70.
El M, Ashour S, Moustafa H, Ahmed I. Altered levels of soluble adhesion molecules in patients with rheumatoid arthritis complicated by peripheral neuropathy. J Rheumatol. 2002;29:57–61.
Witkowska AM, Kuryliszyn-Moskal A, Borawska MH, Hukalowicz K, Markiewicz R. A study on soluble intercellular adhesion molecule-1 and selenium in patients with rheumatoid arthritis complicated by vasculitis. Clin Rheumatol. 2003;22:414–9.
Ates A, Kinikli G, Turgay M, Duman M. Serum-soluble selectin levels in patients with rheumatoid arthritis and systemic sclerosis. Scand J Immunol. 2004;59:315–20.
Sharaki OA, El-Guiziry DA, Abou-Zeid AA, El-Noueam KI, Helal AE, Gaballah AE. Clinical usefulness of basic fibroblast growth factor and E-selectin in patients with rheumatoid arthritis. Egypt J Immunol. 2004;11:91–100.
Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R634–43.
Macias I, Garcia-Perez S, Ruiz-Tudela M, Medina F, Chozas N, Giron-Gonzalez JA. Modification of pro- and antiinflammatory cytokines and vascular-related molecules by tumor necrosis factor—a blockade in patients with rheumatoid arthritis. J Rheumatol. 2005;32:2102–8.
Navarro-Hernandez RE, Oregon-Romero E, Rangel-Villalobos H, Vazquez-Del Mercado M, Ruiz-Quezada SL, Maldonado-Gonzalez M, et al. sE-Selectin expression and A561C polymorphism in relation to rheumatoid arthritis clinical activity. J Rheumatol. 2006;33:1968–72.
Pahor A, Hojs R, Gorenjak M, Rozman B. Accelerated atherosclerosis in pre-menopausal female patients with rheumatoid arthritis. Rheumatol Int. 2006;27:119–23.
Kao AH, Wasko MC, Krishnaswami S, Wagner J, Edmundowicz D, Shaw P, et al. C-reactive protein and coronary artery calcium in asymptomatic women with systemic lupus erythematosus or rheumatoid arthritis. Am J Cardiol. 2008;102:755–60.
Pamuk GE, Vural O, Turgut B, Demir M, Pamuk ON, Cakir N. Increased platelet activation markers in rheumatoid arthritis: are they related with subclinical atherosclerosis? Platelets. 2008;19:146–54.
Wallberg-Jonsson S, Caidahl K, Klintland N, Nyberg G, Rantapaa-Dahlqvist S. Increased arterial stiffness and indication of endothelial dysfunction in long-standing rheumatoid arthritis. Scand J Rheumatol. 2008;37:1–5.
Foster W, Shantsila E, Carruthers D, Lip GY, Blann AD. Circulating endothelial cells and rheumatoid arthritis: relationship with plasma markers of endothelial damage/dysfunction. Rheumatology (Oxford). 2009;48:285–8.
Navarro-Hernandez RE, Oregon-Romero E, Vazquez-Del Mercado M, Rangel-Villalobos H, Palafox-Sanchez CA, Munoz-Valle JF. Expression of ICAM1 and VCAM1 serum levels in rheumatoid arthritis clinical activity. Association with genetic polymorphisms. Dis Markers. 2009;26:119–26.
Pemberton PW, Ahmad Y, Bodill H, Lokko D, Hider SL, Yates AP, et al. Biomarkers of oxidant stress, insulin sensitivity and endothelial activation in rheumatoid arthritis: a cross-sectional study of their association with accelerated atherosclerosis. BMC Res Notes. 2009;2:83.
Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–5.
Olewicz-Gawlik A, Trzybulska D, Grala P, Hrycaj P. Blood serum levels of amino-terminal pro-C-type natriuretic peptide in patients with rheumatoid arthritis. Adv Med Sci. 2010;55:261–5.
Sodergren A, Karp K, Boman K, Eriksson C, Lundstrom E, Smedby T, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther. 2010;12:R158.
de Groot L, Hinkema H, Westra J, Smit AJ, Kallenberg CG, Bijl M, Posthumus MD. Advanced glycation endproducts are increased in rheumatoid arthritis patients with controlled disease. Arthritis Res Ther. 2011;13:R205.
Santos MJ, Carmona-Fernandes D, Canhao H, Canas da Silva J, Fonseca JE, Gil V. Early vascular alterations in SLE and RA patients—a step towards understanding the associated cardiovascular risk. PLoS ONE. 2012;7:e44668.
Klimek E, Skalska A, Kwasny-Krochin B, Surdacki A, Sulicka J, Korkosz M, et al. Differential associations of inflammatory and endothelial biomarkers with disease activity in rheumatoid arthritis of short duration. Mediators Inflamm. 2014;2014:681635.
Pamuk ON, Tozkir H, Uyanik MS, Gurkan H, Saritas F, Duymaz J, et al. PECAM-1 gene polymorphisms and soluble PECAM-1 level in rheumatoid arthritis and systemic lupus erythematosus patients: any link with clinical atherosclerotic events? Clin Rheumatol. 2014;33:1737–43.
Rodriguez-Carrio J, Alperi-Lopez M, Lopez-Mejias R, Lopez P, Ballina-Garcia FJ, Abal F, et al. Antibodies to paraoxonase 1 are associated with oxidant status and endothelial activation in rheumatoid arthritis. Clin Sci (Lond). 2016;130:1889–99.
Sarithala VJ, Koroy P, Yagoda A. Variations in levels of intercellular adhesion molecule-1 with disease course, in rheumatoid arthritis patients. J Clin Diagn Res. 2018;2018:1.
Bezuidenhout JA, Venter C, Roberts TJ, Tarr G, Kell DB, Pretorius E. Detection of citrullinated fibrin in plasma clots of rheumatoid arthritis patients and its relation to altered structural clot properties, disease-related inflammation and prothrombotic tendency. Front Immunol. 2020;11:577523.
Salem HR, Zahran ES. Vascular cell adhesion molecule-1 in rheumatoid arthritis patients: Relation to disease activity, oxidative stress, and systemic inflammation. Saudi Med J. 2021;42:620–8.
Gerasimova EV, Popkova TV, Gerasimova DA, Markina YV, Kirichenko TV. Subclinical carotid atherosclerosis in patients with rheumatoid arthritis at low cardiovascular risk. Biomedicines. 2023;11:1.
Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101.
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–27.
Herrero-Fernandez B, Gomez-Bris R, Somovilla-Crespo B, Gonzalez-Granado JM. Immunobiology of atherosclerosis: a complex net of interactions. Int J Mol Sci. 2019;20:1.
Chi Z, Melendez AJ. Role of cell adhesion molecules and immune-cell migration in the initiation, onset and development of atherosclerosis. Cell Adh Migr. 2007;1:171–5.
Rosenfeld ME, Yla-Herttuala S, Lipton BA, Ord VA, Witztum JL, Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300.
Williams KJ, Tabas I. Lipoprotein retention–and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–40.
Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41.
Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–38.
Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–92.
Harrison M, Smith E, Ross E, Krams R, Segers D, Buckley CD, et al. The role of platelet-endothelial cell adhesion molecule-1 in atheroma formation varies depending on the site-specific hemodynamic environment. Arterioscler Thromb Vasc Biol. 2013;33:694–701.
Kisucka J, Chauhan AK, Zhao BQ, Patten IS, Yesilaltay A, Krieger M, Wagner DD. Elevated levels of soluble P-selectin in mice alter blood-brain barrier function, exacerbate stroke, and promote atherosclerosis. Blood. 2009;113:6015–22.
Ding Z, Issekutz TB, Downey GP, Waddell TK. L-selectin stimulation enhances functional expression of surface CXCR4 in lymphocytes: implications for cellular activation during adhesion and migration. Blood. 2003;101:4245–52.
Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992;89:9991–5.
Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, De Caterina R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ Res. 2000;87:19–25.
Leone M, Boutiere-Albanese B, Valette S, Camoin-Jau L, Barrau K, Albanese J, et al. Cell adhesion molecules as a marker reflecting the reduction of endothelial activation induced by glucocorticoids. Shock. 2004;21:311–4.
Zielinska KA, Van Moortel L, Opdenakker G, De Bosscher K, Van den Steen PE. Endothelial response to glucocorticoids in inflammatory diseases. Front Immunol. 2016;7:592.
Cronstein BN, Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993;36:147–57.
Williams TJ, Hellewell PG. Endothelial cell biology. Adhesion molecules involved in the microvascular inflammatory response. Am Rev Respir Dis. 1992;146:S45-50.
Gonzalez-Amaro R, Diaz-Gonzalez F, Sanchez-Madrid F. Adhesion molecules in inflammatory diseases. Drugs. 1998;56:977–88.
Miller MA, Cappuccio FP. Cellular adhesion molecules and their relationship with measures of obesity and metabolic syndrome in a multiethnic population. Int J Obes (Lond). 2006;30:1176–82.
Patel RB, Colangelo LA, Bielinski SJ, Larson NB, Ding J, Allen NB, et al. Circulating vascular cell adhesion molecule-1 and incident heart failure: the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc. 2020;9:e019390.
Steffen BT, Steffen LM, Tracy R, Siscovick D, Jacobs D, Liu K, et al. Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the multi-ethnic study of atherosclerosis (MESA). Eur J Clin Nutr. 2012;66:600–5.
Jaumdally RJ, Varma C, Blann AD, Macfadyen RJ, Lip GY. Indices of angiogenesis, platelet activation, and endothelial damage/dysfunction in relation to ethnicity and coronary artery disease: differences in central versus peripheral levels. Ann Med. 2007;39:628–33.
Makin AJ, Chung NA, Silverman SH, Lip GY. Thrombogenesis and endothelial damage/dysfunction in peripheral artery disease. Relationship to ethnicity and disease severity. Thromb Res. 2003;111:221–6.
Stepanikova I, Bateman LB, Oates GR. Systemic inflammation in midlife: race, socioeconomic status, and perceived discrimination. Am J Prev Med. 2017;52:S63–76.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding was available for this study.
Author information
Authors and Affiliations
Contributions
Study conception: AZ, AAM. Data collection and analysis: AZ. Data interpretation: AZ, AAM. Writing—first draft: AAM. Writing—review and editing: AZ, AAM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mangoni, A.A., Zinellu, A. A systematic review and meta-analysis of circulating adhesion molecules in rheumatoid arthritis. Inflamm. Res. 73, 305–327 (2024). https://doi.org/10.1007/s00011-023-01837-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01837-6