Abstract
Objective
GTP cyclohydrolase 1(GCH1) was reported to protect against ferroptosis. However, it is not clear whether GCH1 reduced lipopolysaccharide (LPS)-induced macrophage polarization and inflammation by inhibition of ferroptosis.
Methods
Bioinformatics analysis was used to screen differential expression genes (DEGs) and obtain the different pathways and biological features. Lasso cox regression analysis with ferroptosis related DEGs was established to screen the most relevant genes for disease risk. LPS induced Raw264.7 macrophage polarization model and GCH1-specific siRNA oligos transfection were performed to confirm the function of GCH1. Immunofluorescence staining, western blot and quantitative real-time PCR were performed to detect the expression of iNOS, CD206, GCH1, IL6, SLC2A6, F4/80, IL1β, TNFα, IL10, GPX4, ACSL4, AMPK and p-AMPK in macrophages. The levels of ROS, SOD, MDA and GSH were detected according to the instructions of the reagent kit, respectively.
Results
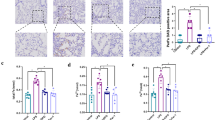
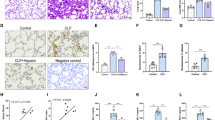
542 DEGs were screened from GSE40885 microarray. GO and KEGG pathway enrichment analysis showed that the upregulated DEGs induced by LPS in alveolar macrophage were closely associated with inflammatory and immune responses, the downregulated DEGs were related to lipid metabolism, insulin resistance and AMPK signal pathway. Lasso cox regression analysis screened GCH1, IL6, and SLC2A6. Our experimental results showed that the expression of GCH1 and IL6 in the LPS group was higher than that in the control group, but there was no difference in the expression of SLC2A6. Bioinformatics analysis with GSE112720 observed that ferroptosis was enriched in GCHfl/fl + LPS group compared with GCHfl/flTie2cre + LPS group and GCHfl/fl + control group. Silence of GCH1 increased the levels of IL6, TNF-α and IL-1β and decreased IL10 level. Silence of GCH1 increased iNOS level and decreased CD206 level. Moreover, silence of GCH1 raised ferroptosis induced by LPS in macrophages and suppressed the activity of AMPK pathway.
Conclusions
GCH1 inhibited ferroptosis in LPS-stimulated macrophages, reduced macrophage toward to M1 polarization and inflammatory response.








Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GCH1:
-
GTP cyclohydrolase-1
- LPS:
-
Lipopolysaccharide
- GPX4:
-
Glutathione peroxidase 4
- ACSL4:
-
Acyl-coenzyme A synthetase long-chain family member 4
- SLC2A6:
-
6 Solute carrier family 2 (facilitated glucose transporter)
- iNOS:
-
Inducible nitric oxide synthase
- CD206:
-
Human mannose receptor 206
- IL6:
-
Interleukin 6
- IL1β:
-
Interleukin 1 beta
- TNFα:
-
Tumor necrosis factor alpha
- IL10:
-
Interleukin 10
- AMPK:
-
AMP-activated protein kinase
- DEG:
-
Differentially expressed gene
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- PCR:
-
Polymerase chain reaction
- DCFH-DA:
-
2',7'-Dichlorodihydrofluorescein diacetate
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- TUNEL:
-
TdT-mediated dUTP nick end-labeling
- DAPI:
-
4',6-Diamidino-2-phenylindole
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- SDS–PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene difluoride
- MODS:
-
Multiple organ dysfunction syndrome
References
Fiest KM, Krewulak KD, Brundin-Mather R, Leia MP, Fox-Robichaud A, Lamontagne F, et al. Patient, public, and healthcare professionals’ sepsis awareness, knowledge, and Information seeking behaviors: a scoping review. Crit Care Med. 2022;50(8):1187–97.
Yang WH, Heithoff DM, Aziz PV, Haslund-Gourley B, Westman JS, Narisawa S, et al. Accelerated aging and clearance of host anti-inflammatory enzymes by discrete pathogens fuels sepsis. Cell Host Microbe. 2018;24(4):500-513e5.
Paudel S, Baral P, Ghimire L, Bergeron S, Jin L, DeCorte JA, et al. CXCL1 regulates neutrophil homeostasis in pneumonia-derived sepsis caused by Streptococcus pneumoniae serotype 3. Blood. 2019;133(12):1335–45.
Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):e1803618.
Rosen DA, Seki SM, Fernandez-Castaneda A, Beiter RM, Eccles JD, Woodfolk JA, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11(478):eaau5266.
Rayes J, Lax S, Wichaiyo S, Watson SK, Di Y, Lombard S, et al. The podoplanin-CLEC-2 axis inhibits inflammation in sepsis. Nat Commun. 2017;8(1):2239.
Savio LEB, de Andrade MP, Figliuolo VR, de Avelar Almeida TF, Santana PT, Oliveira SDS, et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J Hepatol. 2017;67(4):716–26.
Solier S, Müller S, Cañeque T, Versini A, Mansart A, Sindikubwabo F, et al. A druggable copper-signalling pathway that drives inflammation. Nature. 2023;617(7960):386–394.
Lorente-Sorolla C, Garcia-Gomez A, Català-Moll F, Toledano V, Ciudad L, Avendaño-Ortiz J, et al. Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis. Genome Med. 2019;11(1):66.
Song Z, Zhang X, Zhang L, Xu F, Tao X, Zhang H, et al. Progranulin plays a central role in host defense during sepsis by promoting macrophage recruitment. Am J Respir Crit Care Med. 2016;194(10):1219–32.
Tirado M, Koss W. Differentiation of mesothelial cells into macrophage phagocytic cells in a patient with clinical sepsis. Blood. 2018;132(13):1460.
Wang S, Liu G, Li Y, Pan Y. Metabolic reprogramming induces macrophage polarization in the tumor microenvironment. Front Immunol. 2022;13:840029.
Jiang JJ, Zhang GF, Zheng JY, Sun JH, Ding SB. Targeting Mitochondrial ROS-Mediated ferroptosis by quercetin alleviates high-fat diet-induced hepatic lipotoxicity. Front Pharmacol. 2022;13:876550.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–82.
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, et al. The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 2020;127:110108.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85.
Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–32.
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–8.
Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–43.
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6(1):41–53.
Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16(12):1351–60.
Cronin SJF, Rao S, Tejada MA, Turnes BL, Licht-Mayer S, Omura T, et al. Phenotypic drug screen uncovers the metabolic GCH1/BH4 pathway as key regulator of EGFR/KRAS-mediated neuropathic pain and lung cancer. Sci Transl Med. 2022;14(660):eabj1531.
Jing X, Huang YW, Jarzembowski J, Shi Y, Konduri GG, Teng RJ. Caffeine ameliorates hyperoxia-induced lung injury by protecting GCH1 function in neonatal rat pups. Pediatr Res. 2017;82(3):483–9.
Teng RJ, Du J, Xu H, Bakhutashvili I, Eis A, Shi Y, et al. Sepiapterin improves angiogenesis of pulmonary artery endothelial cells with in utero pulmonary hypertension by recoupling endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2011;301(3):L334–45.
Reynier F, de Vos AF, Hoogerwerf JJ, Bresser P, van der Zee JS, Paye M, et al. Gene expression profiles in alveolar macrophages induced by lipopolysaccharide in humans. Mol Med. 2012;18(1):1303–11.
McNeill E, Crabtree MJ, Sahgal N, Patel J, Chuaiphichai S, Iqbal AJ, et al. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic Biol Med. 2015;79:206–16.
Hu J, Qiu D, Yu A, Hu J, Deng H, Li H, et al. YTHDF1 Is a potential pan-cancer biomarker for prognosis and immunotherapy. Front Oncol. 2021;11:607224.
Shen W, Song Z, Zhong X, Huang M, Shen D, Gao P, et al. Sangerbox: a comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta. 2022;1(3): e36.
Zheng Y, Gao W, Zhang Q, Cheng X, Liu Y, Qi Z, et al. Ferroptosis and autophagy-related genes in the pathogenesis of ischemic cardiomyopathy. Front Cardiovasc Med. 2022;9:906753.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–56.
Wang W, Zhu L, Li H, Ren W, Zhuo R, Feng C, et al. Alveolar macrophage-derived exosomal tRF-22–8BWS7K092 activates Hippo signaling pathway to induce ferroptosis in acute lung injury. Int Immunopharmacol. 2022;107:108690.
Young R, Bush SJ, Lefevre L, McCulloch MEB, Lisowski ZM, Muriuki C, et al. Species-specific transcriptional regulation of genes involved in nitric oxide production and arginine metabolism in macrophages. Immunohorizons. 2018;2(1):27–37.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–40.
Yang Y, Wang Y, Guo L, Gao W, Tang TL, Yan M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13(4):355.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62.
Lyu T, Tian C, Tan T, Lyu J, Yan K, Zhao X, et al. AMP-activated protein kinase(AMPK) channel: a global bibliometric analysis from 2012 to 2021. Channels (Austin). 2022;16(1):60–71.
Ma Y, Elmhadi M, Wang C, Li Z, Zhang H, He B, et al. Thiamine Supplementation alleviates lipopolysaccharide-triggered adaptive inflammatory response and modulates energy state via suppression of NFκB/p38 MAPK/AMPK signaling in rumen epithelial cells of goats. Antioxidants (Basel). 2022;11(10):2048.
Griffiths HR, Gao D, Pararasa C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017;12:50–7.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–25.
Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–6.
Uciechowski P, Dempke WC. Interleukin-6: a masterplayer in the cytokine network. Oncology. 2020;98(3):131–7.
Kraft VA, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2019;6(1):41–53.
Xu L, Liu Y, Chen X, Zhong H, Wang Y. Ferroptosis in life: to be or not to be. Biomed Pharmacother. 2023;159:114241.
Lv Y, Wu M, Wang Z, Wang J. Ferroptosis: from regulation of lipid peroxidation to the treatment of diseases. Cell Biol Toxicol. 2022;39(3):827–851.
Gu Y, Li Y, Wang J, Zhang L, Zhang J, Wang Y. Targeting ferroptosis: paving new roads for drug design and discovery. Eur J Med Chem. 2023;247:115015.
Lai K, Song C, Gao M, Deng Y, Lu Z, Li N, et al. Uridine alleviates sepsis-induced acute lung injury by inhibiting ferroptosis of macrophage. Int J Mol Sci. 2023;24(6):5093.
He R, Liu B, Xiong R, Geng B, Meng H, Lin W, et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022;8(1):43.
Hu Q, Lyon CJ, Fletcher JK, Tang W, Wan M, Hu TY. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm Sin B. 2021;11(6):1493–512.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2020YFC2005800), the National Natural Science Foundation of China (No. 82100308), the National Natural Science Foundation Excellent Youth Cultivation Project (No. 20202ZDB01017), the Natural Science Foundation of Jiangxi Province (No. 20202BABL216035, No. 20202BAB206042, No. 20212BCD42005 and G/Y2428).
Author information
Authors and Affiliations
Contributions
LLY and SSC designed the study. YHX performed the experiments. YY and YHY collected the data, and YY, YHY, BL and ZYD analyzed and interpreted the data. LLY, SSC and JL prepared the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study was supported by the Medical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University.
Consent for publication
Not applicable.
Additional information
Responsible Editor: L Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Yuan, Y., Yang, Y. et al. GCH1 reduces LPS-induced alveolar macrophage polarization and inflammation by inhibition of ferroptosis. Inflamm. Res. 72, 1941–1955 (2023). https://doi.org/10.1007/s00011-023-01785-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01785-1