Abstract
Objective
Proper inflammation resolution is crucial to prevent runaway inflammation during sepsis and reduce sepsis-related mortality/morbidity. Previous studies suggest that deleting TRAM, a key TLR4 signaling adaptor, can reprogram the first inflammatory responder cell-neutrophil from an inflammatory state to a resolving state. In this study, we aim to examine the therapeutic potential of TRAM-deficient neutrophils in vivo with recipient mice undergoing experimental sepsis.
Material and methods
Wild-type or Tram−/− mice were intraperitoneally injected with cecal slurry to induce either severe or mild sepsis. Phenotypic examinations of sepsis and neutrophil characteristics were examined in vivo and ex vivo. The propagations of resolution from donor neutrophils to recipient cells such as monocytes, T cells, and endothelial cells were examined through co-culture assays in vitro. The efficacies of Tram−/− neutrophils in reducing inflammation were studied by transfusing either wild-type or Tram−/− neutrophils into septic recipient mice.
Results
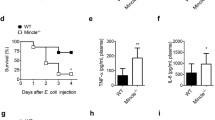
Tram−/− septic mice had improved survival and attenuated injuries within the lung and kidney tissues as compared to wild-type septic mice. Wild-type septic mice transfused with Tram−/− resolving neutrophils exhibited reduced multi-organ damages and improved cellular homeostasis. In vitro co-culture studies revealed that donor Tram−/− neutrophils can effectively propagate cellular homeostasis to co-cultured neighboring monocytes, neutrophils, T cells as well as endothelial cells.
Conclusions
Neutrophils with TRAM deletion render effective reprogramming into a resolving state beneficial for ameliorating experimental sepsis, with therapeutic potential in propagating cellular and tissue homeostasis as well as treating sepsis.





Similar content being viewed by others
Data availability
scRNAseq data were deposited at the NCBI Genebank with the accession number GSE230241. All other experimental data are presented and described in full within this manuscript.
References
Rudd KE, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/S0140-6736(19)32989-7.
da Silva Ramos FJ, de Freitas FGR, Machado FR. Sepsis in patients hospitalized with coronavirus disease 2019: how often and how severe? Curr Opin Crit Care. 2021;27(5):474–9. https://doi.org/10.1097/MCC.0000000000000861.
Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12(2):130–40. https://doi.org/10.1007/s11897-014-0247-z.
Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33(6):498–510. https://doi.org/10.3109/08830185.2014.889129.
Schortgen F, Asfar P. Update in sepsis and acute kidney injury 2014. Am J Respir Crit Care Med. 2015;191(11):1226–31. https://doi.org/10.1164/rccm.201502-0307UP.
Suda K, et al. Neutrophil elastase inhibitor improves survival of rats with clinically relevant sepsis. Shock. 2010;33(5):526–31. https://doi.org/10.1097/SHK.0b013e3181cc064b.
Li G, et al. The neutrophil elastase inhibitor, sivelestat, attenuates sepsis-related kidney injury in rats. Int J Mol Med. 2016;38(3):767–75. https://doi.org/10.3892/ijmm.2016.2665.
Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28(2):137–45. https://doi.org/10.1016/j.smim.2016.03.007.
El Kebir D, Filep JG. Role of neutrophil apoptosis in the resolution of inflammation. Sci World J. 2010;10:1731–48. https://doi.org/10.1100/tsw.2010.169.
Lin R, Yi Z, Wang J, Geng S, Li L. Generation of resolving memory neutrophils through pharmacological training with 4-PBA or genetic deletion of TRAM. Cell Death Dis. 2022;13(4):345. https://doi.org/10.1038/s41419-022-04809-6.
Uddin M, Levy BD. Resolvins: natural agonists for resolution of pulmonary inflammation. Prog Lipid Res. 2011;50(1):75–88. https://doi.org/10.1016/j.plipres.2010.09.002.
Casulli J, et al. CD200R deletion promotes a neutrophil niche for Francisella tularensis and increases infectious burden and mortality. Nat Commun. 2019;10(1):2121. https://doi.org/10.1038/s41467-019-10156-6.
Herrera BS, et al. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect Immun. 2015;83(2):792–801. https://doi.org/10.1128/IAI.02444-14.
Lin R, Zhang Y, Pradhan K, Li L. TICAM2-related pathway mediates neutrophil exhaustion. Sci Rep. 2020;10(1):14397. https://doi.org/10.1038/s41598-020-71379-y.
Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE. 2014;9(12): e115705. https://doi.org/10.1371/journal.pone.0115705.
Steele AM, Starr ME, Saito H. Late therapeutic intervention with antibiotics and fluid resuscitation allows for a prolonged disease course with high survival in a severe murine model of sepsis. Shock. 2017;47(6):726–34. https://doi.org/10.1097/SHK.0000000000000799.
Nowak JE, Harmon K, Caldwell CC, Wong HR. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med. 2012;13(5):e323–9. https://doi.org/10.1097/PCC.0b013e31824fbd90.
Pradhan K, Geng S, Zhang Y, Lin RC, Li L. TRAM-related TLR4 pathway antagonized by IRAK-M mediates the expression of adhesion/coactivating molecules on low-grade inflammatory monocytes. J Immunol. 2021;206(12):2980–8. https://doi.org/10.4049/jimmunol.2000978.
Han H, Ziegler SF. Bronchoalveolar lavage and lung tissue digestion. Bio Protoc. 2013. https://doi.org/10.21769/bioprotoc.859.
Halliday N, et al. CD86 is a selective CD28 ligand supporting FoxP3+ regulatory T cell homeostasis in the presence of high levels of CTLA-4. Front Immunol. 2020;11: 600000.
Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. https://doi.org/10.1164/ajrccm.151.2.7842182.
Sun X, et al. Sphingosine-1–phosphate receptor–3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012;47(5):628–36. https://doi.org/10.1165/rcmb.2012-0048oc.
Murakami K, et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS ONE. 2014;9(9): e106792. https://doi.org/10.1371/journal.pone.0106792.
van der Vlist M, et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron. 2022;110(4):613-6266.e9. https://doi.org/10.1016/j.neuron.2021.11.020.
Pradhan K, Yi Z, Geng S, Li L. Development of exhausted memory monocytes and underlying mechanisms. Front Immunol. 2021;12: 778830. https://doi.org/10.3389/fimmu.2021.778830.
Kusaczuk M, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyric Acid: simple structure-multiple effects. Curr Pharm Des. 2015;21(16):2147–66.
Liu N, Qiang W, Kuang X, Thuillier P, Lynn WS, Wong PK. The peroxisome proliferator phenylbutyric acid (PBA) protects astrocytes from ts 1 MoMuLV-induced oxidative cell death. J Neurovirol. 2002;8(4):318–25.
Farr RL, Lismont C, Terlecky SR, Fransen M. Peroxisome biogenesis in mammalian cells: the impact of genes and environment. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2016;1863(5):1049–60.
Rungsung S, et al. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333–43. https://doi.org/10.1016/j.cyto.2018.03.042.
Kong Q, Wu X, Qiu Z, Huang Q, Xia Z, Song X. Protective effect of dexmedetomidine on acute lung injury via the upregulation of tumour necrosis factor-alpha-induced protein-8-like 2 in septic mice. Inflammation. 2020;43(3):833–46. https://doi.org/10.1007/s10753-019-01169-w.
Mundi S, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res. 2018;114(1):35–52. https://doi.org/10.1093/cvr/cvx226.
Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol. 2015;6:365. https://doi.org/10.3389/fphys.2015.00365.
Cangara HM, et al. Role of endothelial cell-selective adhesion molecule in hematogeneous metastasis. Microvasc Res. 2010;80(1):133–41. https://doi.org/10.1016/j.mvr.2010.02.006.
Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett. 1995;374(3):323–6. https://doi.org/10.1016/0014-5793(95)01110-z.
Suzuki K, et al. Neutrophil elastase damages the pulmonary endothelial glycocalyx in lipopolysaccharide-induced experimental endotoxemia. Am J Pathol. 2019;189(8):1526–35. https://doi.org/10.1016/j.ajpath.2019.05.002.
Ushakumari CJ, et al. Neutrophil elastase increases vascular permeability and leukocyte transmigration in cultured endothelial cells and obese mice. Cells. 2022. https://doi.org/10.3390/cells11152288.
Grechowa I, Horke S, Wallrath A, Vahl CF, Dorweiler B. Human neutrophil elastase induces endothelial cell apoptosis by activating the PERK-CHOP branch of the unfolded protein response. FASEB J. 2017;31(9):3868–81. https://doi.org/10.1096/fj.201700012R.
Ishii T, et al. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol. 2010;177(4):1665–73. https://doi.org/10.2353/ajpath.2010.090793.
Lu RJ, et al. Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex and age-related functional regulation. Nat Aging. 2021;1(8):715–33. https://doi.org/10.1038/s43587-021-00086-8.
Gupta S, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci U S A. 2020;117(28):16481–91. https://doi.org/10.1073/pnas.2003603117.
Spitzer J. Gender differences in some host defense mechanisms. Lupus. 1999;8(5):380–3.
Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88(9):4711–20. https://doi.org/10.1128/JVI.02081-13.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. https://doi.org/10.1038/nri.2016.90.
Acknowledgements
This study was supported partly by the National Institute of Health grants R01 AI172133.
Funding
National Institutes of Health, NIH AI172133.
Author information
Authors and Affiliations
Contributions
LL and RL designed the analyses. RL, JW, YW, and YZ performed the experiment. RL, ZY, and LL analyzed the data. LL and RL wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest related to this publication.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, R., Wang, J., Wu, Y. et al. Resolving neutrophils due to TRAM deletion renders protection against experimental sepsis. Inflamm. Res. 72, 1733–1744 (2023). https://doi.org/10.1007/s00011-023-01779-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01779-z