Abstract
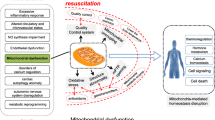
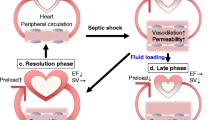
Sepsis is a systemic inflammatory response that follows bacterial infection. Cardiac dysfunction is an important consequence of sepsis that affects mortality and has been attributed to either elevated inflammation or suppression of both fatty acid and glucose oxidation and eventual ATP depletion. Moreover, cardiac adrenergic signaling is compromised in septic patients and this aggravates further heart function. While anti-inflammatory therapies are important for the treatment of the disease, administration of anti-inflammatory drugs did not improve survival in septic patients. This review article summarizes findings on inflammatory and other mechanisms that are triggered in sepsis and affect cardiac function and mortality. Particularly, it focuses on the effects of the disease in metabolic pathways, as well as in adrenergic signaling and the potential interplay of the latter with inflammation. It is suggested that therapeutic approaches should include combination of anti-inflammatory treatments, stimulation of energy production, and restoration of adrenergic signaling in the heart.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–61.
Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10.
Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78.
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6.
Zaky A, Deem S, Bendjelid K, Treggiari MM. Characterization of cardiac dysfunction in sepsis: an ongoing challenge. Shock. 2014;41:12–24.
Ren J, Wu S. A burning issue: do sepsis and systemic inflammatory response syndrome (SIRS) directly contribute to cardiac dysfunction? Front Biosci. 2006;11:15–22.
Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3–11.
Court O, Kumar A, Parrillo JE. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–8.
Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392–7.
Landesberg G, Gilon D, Meroz Y, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33:895–903.
Kumar A, Thota V, Dee L, et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–58.
Hoffmann JN, Werdan K, Hartl WH, et al. Hemofiltrate from patients with severe sepsis and depressed left ventricular contractility contains cardiotoxic compounds. Shock. 1999;12:174–80.
Natanson C, Eichenholz PW, Danner RL, et al. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989;169:823–32.
Finkel MS, Oddis CV, Jacob TD, et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9.
Stein B, Frank P, Schmitz W, et al. Endotoxin and cytokines induce direct cardiodepressive effects in mammalian cardiomyocytes via induction of nitric oxide synthase. J Mol Cell Cardiol. 1996;28:1631–9.
Schulz R, Panas DL, Catena R, et al. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br J Pharmacol. 1995;114:27–34.
Zhong J, Hwang TC, Adams HR, Rubin LJ. Reduced L-type calcium current in ventricular myocytes from endotoxemic guinea pigs. Am J Physiol. 1997;273:H2312–24.
Goldhaber JI, Kim KH, Natterson PD, et al. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am J Physiol. 1996;271:H1449–55.
de Montmollin E, Aboab J, Mansart A, Annane D. Bench-to-bedside review: beta-adrenergic modulation in sepsis. Crit Care. 2009;13:230.
Drosatos K, Drosatos-Tampakaki Z, Khan R, et al. Inhibition of C-JUN-N-terminal kinase increases cardiac PPAR{alpha} expression and fatty acid oxidation and prevents LPS-induced heart dysfunction. J Biol Chem. 2011;286:36331–9.
Drosatos K, Khan RS, Trent CM, et al. Peroxisome proliferator-activated receptor-gamma activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6:550–62.
Schilling J, Lai L, Sambandam N, et al. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor {gamma} coactivator-1 signaling. Circ Heart Fail. 2011;4:474–82. This study showed that activation of PGC-1, a transcriptional co-activator of PPARs, which promotes cardiac fatty acid oxidation and mitochondrial biogenesis, prevents septic cardiac dysfunction.
Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–78.
Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–52.
Docke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–81.
Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–8.
Venet F, Foray AP, Villars-Mechin A, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–81.
Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediat Inflamm. 2013;2013:165974.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74.
Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273–87.
Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–77.
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80.
Zuckerman SH, Evans GF, Guthrie L. Transcriptional and post-transcriptional mechanisms involved in the differential expression of LPS-induced IL-1 and TNF mRNA. Immunology. 1991;73:460–5.
Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci U S A. 1996;93:2774–8.
Sanghera JS, Weinstein SL, Aluwalia M, et al. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–65.
Ebach DR, Riehl TE, Stenson WF. Opposing effects of tumor necrosis factor receptor 1 and 2 in sepsis due to cecal ligation and puncture. Shock. 2005;23:311–8.
Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–71.
Redl H, Schlag G, Paul E, et al. Endogenous modulators of TNF and IL-1 response are under partial control of TNF in baboon bacteremia. Am J Physiol. 1996;271:R1193–8.
Bengtsson A, Redl H, Schlag G, et al. Effects on complement activation and cytokine (TNF-alpha and IL-8) release of infusion of anti-TNF-antibodies or a xanthine derivative (HWA 138) in septic baboons. Acta Anaesthesiol Scand. 1996;40:244–9.
Hinshaw LB, Tekamp-Olson P, Chang AC, et al. Survival of primates in LD100 septic shock following therapy with antibody to tumor necrosis factor (TNF alpha). Circ Shock. 1990;30:279–92.
Fisher Jr CJ, Opal SM, Dhainaut JF, et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. The CB0006 sepsis syndrome study group. Crit Care Med. 1993;21:318–27.
Dhainaut JF, Vincent JL, Richard C, et al. CDP571, a humanized antibody to human tumor necrosis factor-alpha: safety, pharmacokinetics, immune response, and influence of the antibody on cytokine concentrations in patients with septic shock. CPD571 sepsis study group. Crit Care Med. 1995;23:1461–9.
Fisher Jr CJ, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The soluble TNF receptor sepsis study group. N Engl J Med. 1996;334:1697–702.
Abraham E, Glauser MP, Butler T, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45–2081 study group. JAMA. 1997;277:1531–8.
Tsujino M, Hirata Y, Imai T, et al. Induction of nitric oxide synthase gene by interleukin-1 beta in cultured rat cardiomyocytes. Circulation. 1994;90:375–83.
Brady AJ, Poole-Wilson PA, Harding SE, Warren JB. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am J Physiol. 1992;263:H1963–6.
Arnalich F, Lopez-Maderuelo D, Codoceo R, et al. Interleukin-1 receptor antagonist gene polymorphism and mortality in patients with severe sepsis. Clin Exp Immunol. 2002;127:331–6.
Aiura K, Gelfand JA, Burke JF, et al. Interleukin-1 (IL-1) receptor antagonist prevents Staphylococcus epidermidis-induced hypotension and reduces circulating levels of tumor necrosis factor and IL-1 beta in rabbits. Infect Immun. 1993;61:3342–50.
Remick DG, Call DR, Ebong SJ, et al. Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit Care Med. 2001;29:473–81.
Opal SM, Fisher Jr CJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group. Crit Care Med. 1997;25:1115–24.
Bone RC, Fisher Jr CJ, Clemmer TP, et al. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–8.
Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–8.
Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–75.
Fisher Jr CJ, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra sepsis syndrome study group. JAMA. 1994;271:1836–43.
Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29:S121–5.
Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605.
Andreu-Ballester JC, Tormo-Calandin C, Garcia-Ballesteros C, et al. Association of gammadelta T cells with disease severity and mortality in septic patients. Clin Vaccine Immunol. 2013;20:738–46.
Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics. 2011;6:273–83.
Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–83.
Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–2.
Xiao RP, Zhu W, Zheng M, et al. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci. 2004;25:358–65.
Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–83.
Bernstein D, Fajardo G, Zhao M, et al. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–9.
Chesley A, Lundberg MS, Asai T, et al. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–9.
Patterson AJ, Zhu W, Chow A, et al. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–8.
Xiao RP, Avdonin P, Zhou YY, et al. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84:43–52.
Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2.
Zhu WZ, Zheng M, Koch WJ, et al. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–12.
Richter W, Day P, Agrawal R, et al. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 2008;27:384–93.
Rengo G, Lymperopoulos A, Koch WJ. Future g protein-coupled receptor targets for treatment of heart failure. Curr Treat Options Cardiovasc Med. 2009;11:328–38.
Lymperopoulos A, Bathgate A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci. 2013;118:297–334.
DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510.
Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. Beta-arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603–7.
Gao H, Sun Y, Wu Y, et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–17.
Annane D, Trabold F, Sharshar T, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med. 1999;160:458–65.
Kovarik MF, Jones SB, Romano FD. Plasma catecholamines following cecal ligation and puncture in the rat. Circ Shock. 1987;22:281–90.
Boldt J, Menges T, Kuhn D, et al. Alterations in circulating vasoactive substances in the critically ill—a comparison between survivors and non-survivors. Intensive Care Med. 1995;21:218–25.
Jones AE, Craddock PA, Tayal VS, Kline JA. Diagnostic accuracy of left ventricular function for identifying sepsis among emergency department patients with nontraumatic symptomatic undifferentiated hypotension. Shock. 2005;24:513–7.
Azimi G, Vincent JL. Ultimate survival from septic shock. Resuscitation. 1986;14:245–53.
Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316.
Hahn PY, Wang P, Tait SM, et al. Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock. 1995;4:269–73.
Gulick T, Chung MK, Pieper SJ, et al. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989;86:6753–7.
Bohm M, Kirchmayr R, Gierschik P, Erdmann E. Increase of myocardial inhibitory G-proteins in catecholamine-refractory septic shock or in septic multiorgan failure. Am J Med. 1995;98:183–6.
Macarthur H, Westfall TC, Riley DP, et al. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci U S A. 2000;97:9753–8.
Hare JM, Loh E, Creager MA, Colucci WS. Nitric oxide inhibits the positive inotropic response to beta-adrenergic stimulation in humans with left ventricular dysfunction. Circulation. 1995;92:2198–203.
Barth E, Radermacher P, Thiemermann C, et al. Role of inducible nitric oxide synthase in the reduced responsiveness of the myocardium to catecholamines in a hyperdynamic, murine model of septic shock. Crit Care Med. 2006;34:307–13.
Lymperopoulos A, Rengo G, Koch WJ. GRK2 inhibition in heart failure: something old, something new. Curr Pharm Des. 2012;18:186–91.
Salazar NC, Vallejos X, Siryk A, et al. GRK2 blockade with betaARKct is essential for cardiac beta2-adrenergic receptor signaling towards increased contractility. Cell Commun Signal. 2013;11:64.
Fan H, Bitto A, Zingarelli B, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–51.
Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–14.
Ibrahim-Zada I, Rhee P, Gomez CT, et al. Inhibition of sepsis-induced inflammatory response by beta1-adrenergic antagonists. J Trauma Acute Care Surg. 2014;76:320–7. discussion 327–8.
Pinsky MR. Is there a role for beta-blockade in septic shock? JAMA. 2013;310:1677–8.
Godlewski G, Schlicker E, Baranowska U, Malinowska B. Recruitment of functionally active heart beta2-adrenoceptors in the initial phase of endotoxic shock in pithed rats. Shock. 2006;26:510–5.
Valen G. Innate immunity and remodelling. Heart Fail Rev. 2011;16:71–8.
Leone M, Boyadjiev I, Boulos E, et al. A reappraisal of isoproterenol in goal-directed therapy of septic shock. Shock. 2006;26:353–7.
Fink T, Heymann P, Taha-Melitz S, et al. Dobutamine pretreatment improves survival, liver function, and hepatic microcirculation after polymicrobial sepsis in rat. Shock. 2013;40:129–35.
Cudmore LA, Muurlink T, Whittem T, Bailey SR. Effects of oral clenbuterol on the clinical and inflammatory response to endotoxaemia in the horse. Res Vet Sci. 2013;94:682–6.
Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–91.
Gouni I, Oka K, Etienne J, Chan L. Endotoxin-induced hypertriglyceridemia is mediated by suppression of lipoprotein lipase at a post-transcriptional level. J Lipid Res. 1993;34:139–46.
Kaufmann RL, Matson CF, Beisel WR. Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis. 1976;133:548–55.
Nogueira AC, Kawabata V, Biselli P, et al. Changes in plasma free fatty acid levels in septic patients are associated with cardiac damage and reduction in heart rate variability. Shock. 2008;29:342–8.
Bagby GJ, Spitzer JA. Lipoprotein lipase activity in rat heart and adipose tissue during endotoxic shock. Am J Physiol. 1980;238:H325–30.
Scholl RA, Lang CH, Bagby GJ. Hypertriglyceridemia and its relation to tissue lipoprotein lipase activity in endotoxemic, Escherichia coli bacteremic, and polymicrobial septic rats. J Surg Res. 1984;37:394–401.
Bagby GJ, Corll CB, Martinez RR. Triacylglycerol kinetics in endotoxic rats with suppressed lipoprotein lipase activity. Am J Physiol. 1987;253:E59–64.
Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–51.
Osorio JC, Stanley WC, Linke A, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–12.
Raymond RM, McLane MP, Law WR, et al. Myocardial insulin resistance during acute endotoxin shock in dogs. Diabetes. 1988;37:1684–8.
Tessier JP, Thurner B, Jungling E, et al. Impairment of glucose metabolism in hearts from rats treated with endotoxin. Cardiovasc Res. 2003;60:119–30.
Dhainaut JF, Huyghebaert MF, Monsallier JF, et al. Coronary hemodynamics and myocardial metabolism of lactate, free fatty acids, glucose, and ketones in patients with septic shock. Circulation. 1987;75:533–41.
Hotchkiss RS, Rust RS, Dence CS, et al. Evaluation of the role of cellular hypoxia in sepsis by the hypoxic marker [18F]fluoromisonidazole. Am J Physiol. 1991;261:R965–72.
Lu B, Moser A, Shigenaga JK, et al. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–41.
Feingold K, Kim MS, Shigenaga J, et al. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. Am J Physiol Endocrinol Metab. 2004;286:E201–7.
Jia L, Takahashi M, Morimoto H, et al. Changes in cardiac lipid metabolism during sepsis: the essential role of very low-density lipoprotein receptors. Cardiovasc Res. 2006;69:545–55.
Read TE, Harris HW, Grunfeld C, et al. The protective effect of serum lipoproteins against bacterial lipopolysaccharide. Eur Heart J. 1993;14(Suppl K):125–9.
Ghoshal S, Witta J, Zhong J, et al. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–7.
Memon RA, Bass NM, Moser AH, et al. Down-regulation of liver and heart specific fatty acid binding proteins by endotoxin and cytokines in vivo. Biochim Biophys Acta. 1999;1440:118–26.
Memon RA, Fuller J, Moser AH, et al. In vivo regulation of acyl-CoA synthetase mRNA and activity by endotoxin and cytokines. Am J Physiol. 1998;275:E64–72.
Uji Y, Yamamoto H, Tsuchihashi H, et al. Adiponectin deficiency is associated with severe polymicrobial sepsis, high inflammatory cytokine levels, and high mortality. Surgery. 2009;145:550–7.
Haraguchi G, Kosuge H, Maejima Y, et al. Pioglitazone reduces systematic inflammation and improves mortality in apolipoprotein E knockout mice with sepsis. Intensive Care Med. 2008;34:1304–12.
Chima RS, Hake PW, Piraino G, et al. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008;36:2849–57.
Siddiqui AM, Cui X, Wu R, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34:1874–82.
Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res. 2005;54:464–70.
Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–9.
Yamauchi T, Kamon J, Waki H, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276:41245–54.
Yang WS, Jeng CY, Wu TJ, et al. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376–80.
Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR gamma 2, but not C/EBP alpha-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308:933–9.
Fang X, Palanivel R, Cresser J, et al. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab. 2010;299:E721–9.
Yoon MJ, Lee GY, Chung JJ, et al. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–70.
Reynolds K, Novosad B, Hoffhines A, et al. Pretreatment with troglitazone decreases lethality during endotoxemia in mice. J Endotoxin Res. 2002;8:307–14.
Kapoor A, Shintani Y, Collino M, et al. Protective role of peroxisome proliferator-activated receptor-beta/delta in septic shock. Am J Respir Crit Care Med. 2010;182:1506–15.
Langley RJ, Tsalik EL, Velkinburgh JC, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. 2013;5:195ra95. This study showed that markers of increased tissue fatty acid oxidation detected in plasma of septic patients predict improved survival in sepsis.
Watts JA, Kline JA, Thornton LR, et al. Metabolic dysfunction and depletion of mitochondria in hearts of septic rats. J Mol Cell Cardiol. 2004;36:141–50.
Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007;28:24–8.
Turdi S, Han X, Huff AF, et al. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: role of autophagy. Free Radic Biol Med. 2012;53:1327–38.
Vanasco V, Magnani ND, Cimolai MC, et al. Endotoxemia impairs heart mitochondrial function by decreasing electron transfer, ATP synthesis and ATP content without affecting membrane potential. J Bioenerg Biomembr. 2012;44:243–52.
Chen HW, Hsu C, Lu TS, et al. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock. 2003;20:274–9.
Piquereau J, Godin R, Deschenes S, et al. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9:1837–51.
Noto A, Giacomini M, Palandi A, et al. Levosimendan in septic cardiac failure. Intensive Care Med. 2005;31:164–5.
Givertz MM, Andreou C, Conrad CH, Colucci WS. Direct myocardial effects of levosimendan in humans with left ventricular dysfunction: alteration of force-frequency and relaxation-frequency relationships. Circulation. 2007;115:1218–24.
Torraco A, Carrozzo R, Piemonte F, et al. Effects of levosimendan on mitochondrial function in patients with septic shock: a randomized trial. Biochimie. 2014;102:166–73.
Compliance with Ethics Guidelines
Conflict of Interest
Konstantinos Drosatos, Anastasios Lymperopoulos, Peter Johannes Kennel, Nina Pollak, P. Christian Schulze, and Ira J. Goldberg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
Drs. Drosatos, Schulze, and Goldberg are supported by grants through the National Heart, Lung, and Blood Institute (NHLBI) (HL112853, KD; HL114813, PCS; HL45095 and HL73029, IJG). Dr. Lymperopoulos is supported by an AHA Scientist Development Grant (No. 09SDG2010138, National Center).
Author information
Authors and Affiliations
Corresponding author
Additional information
Anastasios Lymperopoulos and Peter Johannes Kennel contributed equally to this review.
This article is part of the Topical Collection on Pathophysiology: Neuroendocrine, Vascular, and Metabolic Factors
Rights and permissions
About this article
Cite this article
Drosatos, K., Lymperopoulos, A., Kennel, P.J. et al. Pathophysiology of Sepsis-Related Cardiac Dysfunction: Driven by Inflammation, Energy Mismanagement, or Both?. Curr Heart Fail Rep 12, 130–140 (2015). https://doi.org/10.1007/s11897-014-0247-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-014-0247-z