Abstract
Objective and design
Circulating enzymatic activity and RAAS regulation in severe cases of COVID-19 remains unclear, therefore we measured the serum activity of several proteases as potential targets to control the SARS-CoV-2 infection.
Material or subjects
152 patients with COVID-19-like symptoms were grouped according to the severity of symptoms (COVID-19 negative, mild, moderate and severe).
Methods
Serum samples of COVID-19 patients and controls were subjected to biochemical analysis and enzymatic assays of ACE2, ACE, DPPIV, PREP and CAT L. One-way ANOVA and multivariate logistic regression analysis were used. Statistical significance was accepted at p < 0.05.
Results
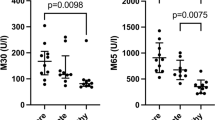
We detected a positive correlation among comorbidities, higher C-reactive protein (CRP) and D-dimer levels with disease severity. Enzymatic assays revealed an increase in serum ACE2 and CAT L activities in severe COVID-19 patients, while ACE, DPPIV and PREP activities were significantly reduced. Notably, analysis of ACE2/ACE activity ratio suggests a possible imbalance of ANG II/ANG(1-7) ratio, in a positive association with the disease severity.
Conclusion
Our findings reveal a correlation between proteases activity and the severity of COVID-19. These enzymes together contribute to the activation of pro-inflammatory pathways, trigger a systemic activation of inflammatory mediators, leading to a RAAS dysregulation and generating a significant damage in several organs, contributing to poor outcomes of severe cases.









Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
References
Hui DS, Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–6.
WHO - World Health Organization. Pneumonia of unknown cause—China (2020, accessed 18 Aug 2022); https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229.
Lu S, Ye Q, Singh D, Cao Y, Diedrich JK, Yates JR, et al. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat Commun. 2021;12:502.
Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q, et al. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6:1–19.
Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–50.
Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, et al. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198:867–77.
Hamming I, Cooper M, Haagmans B, Hooper N, Korstanje R, Osterhaus A, et al. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1–11.
Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss H-P, et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–7.
Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–28.
Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–19.
Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–21.
Gaddam R, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy-Drug Targets. 2014;13:224–34.
Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, et al. Tumor necrosis factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem. 2005;280:30113–9.
Guo X-M, Cao J, Cai J-P, Wu J, Huang J, Asthana P, et al. Control of SARS-CoV-2 infection by MT1-MMP-mediated shedding of ACE2. Nat Commun. 2022;2022:13.
Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2010;85:873–82.
Ramos SG, Rattis BAC, Ottaviani G, Celes MRN, Dias EP. ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients. Am J Pathol. 2021;191:1154–64.
Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol-Lung Cell Mol Physiol. 2018;314:L17-31.
Verano-Braga T, Martins AL, Motta-Santos D, Campagnole-Santos M, Santos RS. ACE2 in the renin–angiotensin system. Clin Sci. 2020;134:3063–78.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6.
Metzger R, Franke FE, Bohle RM, François A-G, Danilov SM. Heterogeneous distribution of angiotensin I-converting enzyme (CD143) in the human and rat vascular systems: Vessel, organ and species specificity. Microvasc Res. 2011;81:206–15.
Turner AJ, Hooper NM. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci. 2002;23:177–83.
Tepasse P-R, Vollenberg R, Steinebrey N, König S. High angiotensin-converting enzyme and low carboxypeptidase N serum activity correlate with disease severity in COVID-19 Patients. J Personal Med. 2022;12:406.
Trzaskalski NA, Fadzeyeva E, Mulvihill EE. Dipeptidyl peptidase-4 at the interface between inflammation and metabolism. Clin Med Insights: Endocrinol Diabetes. 2020. https://doi.org/10.1177/1179551420912972.
Penttinen A, Tenorio-Laranga J, Siikanen A, Morawski M, Roßner S, Arturo G-H. Prolyl oligopeptidase: a rising star on the stage of neuroinflammation research. CNS Neurol Disord Drug Targets. 2011;10:340–8.
Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019.
Kahne T, Lendeckel U, Wrenger S, Neubert K, Ansorge S, Reinhold D. Dipeptidyl peptidase IV: a cell surface peptidase involved in regulating T cell growth (review). Int J Mol Med. 1999;4:3–15.
Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–31.
García-Horsman JA, Männistö PT, Venäläinen JI. On the role of prolyl oligopeptidase in health and disease. Neuropeptides. 2007;41:1–24.
Bassendine MF, Bridge SH, McCaughan GW, Gorrell MD. COVID-19 and comorbidities: a role for dipeptidyl peptidase 4 (DPP4) in disease severity? J Diabetes. 2020;12:649–58.
Yang Y, Cai Z, Zhang J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: a meta-analysis. Ashraf GM, editor. PLoS ONE. 2021;16:e0251916.
Nádasdi Á, Sinkovits G, Bobek I, Lakatos B, Förhécz Z, Prohászka ZZ, et al. Decreased circulating dipeptidyl peptidase-4 enzyme activity is prognostic for severe outcomes in COVID-19 inpatients. Biomark Med. 2022;16:317–30.
Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci. 2005;102:11876–81.
Huang I-C, Bosch BJ, Li F, Li W, Lee KH, Ghiran S, et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J Biol Chem. 2006;281:3198–203.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta BBA Proteins Proteom. 2012;1824:68–88.
Fonović M, Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochem Biophys Acta. 2014;1840:2560–70.
Vidak E, Javoršek U, Vizovišek M, Turk B. Cysteine cathepsins and their extracellular roles: shaping the microenvironment. Cells. 2019;8:264.
Zhao M-M, Yang W-L, Yang F-Y, Zhang L, Huang W-J, Hou W, et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct Target Ther. 2021;6:134.
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Detection of, et al. novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2019;2020:25.
Pedersen KB, Sriramula S, Chhabra KH, Xia H, Lazartigues E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol-Regulat Integr Compar Physiol. 2011;301:R1293–9.
Kim YB, Kopcho LM, Kirby MS, Hamann LG, Weigelt CA, Metzler WJ, et al. Mechanism of Gly-Pro-pNA cleavage catalyzed by dipeptidyl peptidase-IV and its inhibition by saxagliptin (BMS-477118). Arch Biochem Biophys. 2006;445:9–18.
Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, et al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020;71:2174–9.
Valerio L, Ferrazzi P, Sacco C, Ruf W, Kucher N, Konstantinides SV, et al. Course of D-dimer and C-reactive protein levels in survivors and nonsurvivors with COVID-19 pneumonia: a retrospective analysis of 577 patients. Thromb Haemost. 2020;121:98–101.
Pagliaro P, Penna C. ACE/ACE2 ratio: a key also in 2019 coronavirus disease (Covid-19)? Front Med. 2020;7:335.
Reindl-Schwaighofer R, Hödlmoser S, Domenig O, Krenn K, Eskandary F, Krenn S, et al. The systemic renin-angiotensin system in COVID-19. Sci Rep. 2022;12:20117.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–9.
Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–99.
Gioia M, Ciaccio C, Calligari P, De Simone G, Sbardella D, Tundo G, et al. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem Pharmacol. 2020. https://doi.org/10.1016/j.bcp.2020.114225.
Alves MHME, Mahnke LC, Macedo TC, dos Silva TK, Carvalho-Junior, LB. The enzymes in COVID-19: a review. Biochimie. 2022;197:38–48.
Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:1–14.
Huang Y, Yang C, Xu X, Xu W, Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–9.
Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–33.
Bollavaram K, Leeman TH, Lee MW, Kulkarni A, Upshaw SG, Yang J, et al. Multiple sites on SARS-CoV-2 spike protein are susceptible to proteolysis by cathepsins B, K, L, S, and V. Protein Sci. 2021;30:1131–43.
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–8.
Úri K, Fagyas M, Kertész A, Borbély A, Jenei C, Bene O, et al. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin-Angiotensin-Aldosterone Syst. 2016. https://doi.org/10.1177/1470320316668435.
Fagyas M, Kertész A, Siket IM, Bánhegyi V, Kracskó B, Szegedi A, et al. Level of the SARS-CoV-2 receptor ACE2 activity is highly elevated in old-aged patients with aortic stenosis: implications for ACE2 as a biomarker for the severity of COVID-19. GeroScience. 2021;43:19–29.
Patel S, Juno J, Lee WS, Wragg K, Hogarth PM, Kent S, et al. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences of COVID-19. J Hypertens. 2021;39: e394.
Fagyas M, Fejes Z, Sütő R, Nagy Z, Székely B, Pócsi M, et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int J Infect Dis. 2022;115:8–16.
Bastolla U, Chambers P, Abia D, Garcia-Bermejo M-L, Fresno M. Is Covid-19 severity associated with ACE2 degradation? Front Drug Discov. 2022. https://doi.org/10.3389/fddsv.2021.789710.
Poudel A, Poudel Y, Adhikari A, Aryal BB, Dangol D, Bajracharya T, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. Ai T, editor. PLoS ONE. 2021;16:e0256744.
Maza MC, Úbeda M, Delgado P, Horndler L, Llamas MA, van Santen HM, et al. ACE2 Serum levels as predictor of infectability and outcome in COVID-19. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.836516.
Montanari M, Canonico B, Nordi E, Vandini D, Barocci S, Benedetti S, et al. Which ones, when and why should renin-angiotensin system inhibitors work against COVID-19? Adv Biol Regul. 2021. https://doi.org/10.1016/j.jbior.2021.100820.
Wang K, Chen W, Zhang Z, Deng Y, Lian J-Q, Du P, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283.
Barnes CO, Jette CA, Abernathy ME, Dam K-MA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:1–6.
Duru CE, Duru IA, Adegboyega AE. In silico identification of compounds from Nigella sativa seed oil as potential inhibitors of SARS-CoV-2 targets. Bull Natl Res Centre. 2021;45:57.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.
Bosch BJ, Bartelink W, Rottier PJM. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol. 2008;82:8887–90.
Mellott DM, Tseng C-T, Drelich A, Fajtová P, Chenna BC, Kostomiris DH, et al. A clinical-stage cysteine protease inhibitor blocks SARS-CoV-2 infection of human and monkey cells. ACS Chem Biol. 2021;16:642–50.
Gomes CP, Fernandes DE, Casimiro F, da Mata GF, Passos MT, Varela P, et al. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Front Cell Infect Microbiol. 2020. https://doi.org/10.3389/fcimb.2020.589505.
Takeda M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol Immunol. 2021. https://doi.org/10.1111/1348-0421.12945.
Wilczynski SA, Wenceslau CF, McCarthy CG, Webb RC. A cytokine/bradykinin storm comparison: what is the relationship between hypertension and COVID-19? Am J Hypertens. 2021;34:304–6.
Carvalho PR, Sirois P, Fernandes PD. The role of kallikrein-kinin and renin-angiotensin systems in COVID-19 infection. Peptides. 2021. https://doi.org/10.1016/j.peptides.2020.170428.
Khan KS, Reed-Embleton H, Lewis J, Bain P, Mahmud S. Angiotensin converting enzyme inhibitors do not increase the risk of poor outcomes in COVID-19 disease. A multi-centre observational study. Scottish Med J. 2020;65:149–53.
Avanoglu Guler A, Tombul N, Aysert Yıldız P, Özger HS, Hızel K, Gulbahar O, et al. The assessment of serum ACE activity in COVID-19 and its association with clinical features and severity of the disease. Scand J Clin Lab Invest. 2021;81:160–5.
Henry BM, Benoit JL, Rose J, de Oliveira MHS, Lippi G, Benoit SW. Serum ACE activity and plasma ACE concentration in patients with SARS-CoV-2 infection. Scand J Clin Lab Invest. 2021;81:272–5.
Karakaş Çelik S, Çakmak Genç G, Pişkin N, Açikgöz B, Altinsoy B, Kurucu İşsiz B, et al. Polymorphisms of ACE (I/D) and ACE2 receptor gene (Rs2106809, Rs2285666) are not related to the clinical course of COVID-19: a case study. J Med Virol. 2021;93:5947–52.
Baştuğ S, Çavdarlı B, Baştuğ A, Şencan İ, Tunçez E, Çakır EY, et al. Are angiotensin converting enzyme (ACE1/ACE2) gene variants associated with the clinical severity of COVID-19 pneumonia? A single-center cohort study. Anatolian J Cardiol. 2022;26:133–40.
Sabater Molina M, Nicolás Rocamora E, Bendicho AI, Vázquez EG, Zorio E, Rodriguez FD, et al. Polymorphisms in ACE, ACE2, AGTR1 genes and severity of COVID-19 disease. Ciccacci C, editor. PLoS ONE. 2022;17:e0263140.
Papadopoulou A, Fragkou PC, Maratou E, Dimopoulou D, Kominakis A, Kokkinopoulou I, et al. Angiotensin-converting-enzyme insertion/deletion polymorphism, ACE activity, and COVID-19: A rather controversial hypothesis. A case-control study. J Med Virol. 2022;94:1050–9.
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Investig. 1990;86:1343–6.
Danser AHJ, Batenburg WW, van den Meiracker AH, Danilov SM. ACE phenotyping as a first step toward personalized medicine for ACE inhibitors. Why does ACE genotyping not predict the therapeutic efficacy of ACE inhibition? Pharmacol Therapeut. 2007;113:607–18.
Castellon R, Hamdi H. Demystifying the ACE polymorphism: from genetics to biology. Curr Pharmaceut Design. 2007;13:1191–8.
Shukla AK, Banerjee M. Angiotensin-converting-enzyme 2 and renin-angiotensin system inhibitors in COVID-19: an update. High Blood Pressure Cardiovasc Prevent. 2021;28:129–39.
South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circul Physiol. 2020;318:H1084–90.
Serfozo P, Wysocki J, Gulua G, Schulze A, Ye M, Liu P, et al. Ang II (angiotensin II) conversion to angiotensin-(1–7) in the circulation Is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75:173–82.
Silva-Aguiar RP, Peruchetti DB, Rocco PRM, Schmaier AH, Silva PMR, Martins MA, et al. Role of the renin-angiotensin system in the development of severe COVID-19 in hypertensive patients. Am J Physiol-Lung Cell Mol Physiol. 2020;319:L596-602.
Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21:20–7.
Sachse A, Wolf G. Angiotensin II–induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–46.
Okamoto H, Ichikawa N. The pivotal role of the angiotensin-II–NF-κB axis in the development of COVID-19 pathophysiology. Hypertens Res. 2021;44:126–8.
Han C, Liu J, Liu X, Li M. Angiotensin II induces C-reactive protein expression through ERK1/2 and JNK signaling in human aortic endothelial cells. Atherosclerosis. 2010;212:206–12.
Waumans Y, Baerts L, Kehoe K, Lambeir A-M, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease. Atherosc Front Immunol. 2015;6:387.
Bracke A, De-Hert E, De-bruyn M, Claesen K, Vliegen G, Vujkovic A, et al. Proline-specific peptidase activities (DPP4, PRCP, FAP and PREP) in plasma of hospitalized COVID-19 patients. Clin Chim Acta. 2022;531:4–11.
Schlicht K, Rohmann N, Geisler C, Hollstein T, Knappe C, Hartmann K, et al. Circulating levels of soluble dipeptidylpeptidase-4 are reduced in human subjects hospitalized for severe COVID-19 infections. Int J Obes. 2020;44:2335–8.
Scheen AJ. DPP-4 inhibition and COVID-19: from initial concerns to recent expectations. Diabetes Metab. 2020. https://doi.org/10.1016/j.diabet.2020.11.005.
Rhee SY, Lee J, Nam H, Kyoung D-S, Shin DW, Kim DJ. Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. Diabetes Metab J. 2021;45:251–9.
Rakhmat II, Kusmala YY, Handayani DR, Juliastuti H, Nawangsih EN, Wibowo A, et al. Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19)—A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2021;15:777–82.
Zein AFMZ, Raffaello WM. Dipeptidyl peptidase-4 (DPP-IV) inhibitor was associated with mortality reduction in COVID-19—a systematic review and meta-analysis. Prim Care Diabetes. 2021;16:162–7.
Funding
This work was supported by grants from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo—2014/27198-8, 2019/05266-5, 2019/01487-7) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico—423165/2021-6).
Author information
Authors and Affiliations
Contributions
Conceptualization: JBP; methodology: RLN, JB, MR, GFM, DEF, JBP; formal analysis and investigation: RLN, JB, JGA, CAB, CPG, MYI; writing—original draft preparation: RLN, JB, MYI, GMK, JBP; writing—review and editing: RLN, JB, MYI, JBP; funding acquisition: MYI, GMK, JBP; resources: MYI, GMK; supervision: JBP.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethics approval
All procedures were conducted in accordance of the principles of Helsinki Declaration. Approval was granted by the Ethics Committee of the Federal University of São Paulo (CAAE 31929120.0.0000.5505). The patients provided their written informed consent to participate in this study and for the publication of any potentially data included in this article.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Responsible Editor: Anatolii Kubyshkin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neves, R.L., Branquinho, J., Arata, J.G. et al. ACE2, ACE, DPPIV, PREP and CAT L enzymatic activities in COVID-19: imbalance of ACE2/ACE ratio and potential RAAS dysregulation in severe cases. Inflamm. Res. 72, 1719–1731 (2023). https://doi.org/10.1007/s00011-023-01775-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01775-3