Abstract
Background
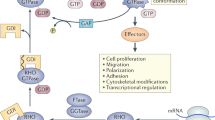
RhoG is a multifaceted member of the Rho family of small GTPases, sharing the highest sequence identity with the Rac subfamily members. It acts as a molecular switch, when activated, plays a central role in regulating the fundamental processes in immune cells, such as actin-cytoskeleton dynamics, transendothelial migration, survival, and proliferation, including immunological functions (e.g., phagocytosis and trogocytosis) during inflammatory responses.
Method
We have performed a literature review based on published original and review articles encompassing the significant effect of RhoG on immune cell functions from central databases, including PubMed and Google Scholar.
Results and conclusions
Recently published data shows that the dynamic expression of different transcription factors, non-coding RNAs, and the spatiotemporal coordination of different GEFs with their downstream effector molecules regulates the cascade of Rho signaling in immune cells. Additionally, alterations in RhoG-specific signaling can lead to physiological, pathological, and developmental adversities. Several mutations and RhoG-modulating factors are also known to pre-dispose the downstream signaling with abnormal gene expression linked to multiple diseases. This review focuses on the cellular functions of RhoG, interconnecting different signaling pathways, and speculates the importance of this small GTPase as a prospective target against several pathological conditions.



Similar content being viewed by others
Data availability
No data were used for the research described in the article.
References
Hervé JC, Bourmeyster N. Rho GTPases at the crossroad of signaling networks in mammals. Small GTPases. 2015;6:43–8.
Colicelli J. Human RAS superfamily proteins and related GTPases. Science's STKE : signal transduction knowledge environment 2004; 2004:Re13.
Nayak RC, Chang KH, Vaitinadin NS, Cancelas JA. Rho GTPases control specific cytoskeleton-dependent functions of hematopoietic stem cells. Immunol Rev. 2013;256:255–68.
Dipankar P, Kumar P, Dash SP, Sarangi PP. Functional and Therapeutic Relevance of Rho GTPases in Innate Immune Cell Migration and Function during Inflammation: An In Silico Perspective. Mediators of inflammation 2021; 2021:6655412.
Haga RB, Ridley AJ. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–21.
Gauthier-Rouvière C, Vignal E, Mériane M, Roux P, Montcourier P, Fort P. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol Biol Cell. 1998;9:1379–94.
Steffen A, Ladwein M, Dimchev GA, Hein A, Schwenkmezger L, Arens S, et al. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci. 2013;126:4572–88.
Vigorito E, Billadeu DD, Savoy D, McAdam S, Doody G, Fort P, et al. RhoG regulates gene expression and the actin cytoskeleton in lymphocytes. Oncogene. 2003;22:330–42.
Katoh H, Hiramoto K, Negishi M. Activation of Rac1 by RhoG regulates cell migration. J Cell Sci. 2006;119:56–65.
Murga C, Zohar M, Teramoto H, Gutkind JS. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207–16.
Vincent S, Jeanteur P, Fort P. Growth-regulated expression of rhoG, a new member of the ras homolog gene family. Mol Cell Biol. 1992;12:3138–48.
Schumacher S, Franke K. miR-124-regulated RhoG: A conductor of neuronal process complexity. Small GTPases. 2013;4:42–6.
Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77.
Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309.
Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9.
Roux P, Gauthier-Rouvière C, Doucet-Brutin S, Fort P. The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Current biology : CB. 1997;7:629–37.
Wennerberg K, Ellerbroek SM, Liu RY, Karnoub AE, Burridge K, Der CJ. RhoG signals in parallel with Rac1 and Cdc42. J Biol Chem. 2002;277:47810–7.
Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–82.
van Rijssel J, Hoogenboezem M, Wester L, Hordijk PL, Van Buul JD. The N-terminal DH-PH domain of Trioinduces cell spreading and migration by regulating lamellipodia dynamics in a Rac1-dependent fashion. PLoS ONE. 2012;7: e29912.
Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouvière C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113(Pt 4):729–39.
Baumeister MA, Rossman KL, Sondek J, Lemmon MA. The Dbs PH domain contributes independently to membrane targeting and regulation of guanine nucleotide-exchange activity. Biochem J. 2006;400:563–72.
Jaiswal M, Dvorsky R, Ahmadian MR. Deciphering the molecular and functional basis of Dbl family proteins: a novel systematic approach toward classification of selective activation of the Rho family proteins. J Biol Chem. 2013;288:4486–500.
Fuentes EJ, Karnoub AE, Booden MA, Der CJ, Campbell SL. Critical role of the pleckstrin homology domain in Dbs signaling and growth regulation. J Biol Chem. 2003;278:21188–96.
Bircher JE, Koleske AJ. (2021) Trio family proteins as regulators of cell migration and morphogenesis in development and disease - mechanisms and cellular contexts. Journal of cell science 134.
Schuebel KE, Movilla N, Rosa JL, Bustelo XR. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–21.
Movilla N, Bustelo XR. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol. 1999;19:7870–85.
Ellerbroek SM, Wennerberg K, Arthur WT, Dunty JM, Bowman DR, DeMali KA, et al. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol Biol Cell. 2004;15:3309–19.
May V, Schiller MR, Eipper BA, Mains RE. Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J Neurosci Off J Soc Neurosci. 2002;22:6980–90.
Kim K, Lee J, Moon H, Lee SA, Kim D, Yang S, et al. (2018) The Intermolecular Interaction of Ephexin4 Leads to Autoinhibition by Impeding Binding of RhoG. Cells 7.
Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, et al. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol. 2010;190:461–77.
Komiya Y, Onodera Y, Kuroiwa M, Nomimura S, Kubo Y, Nam JM, et al. The Rho guanine nucleotide exchange factor ARHGEF5 promotes tumor malignancy via epithelial-mesenchymal transition. Oncogenesis. 2016;5: e258.
Wang Z, Kumamoto Y, Wang P, Gan X, Lehmann D, Smrcka AV, et al. Regulation of immature dendritic cell migration by RhoA guanine nucleotide exchange factor Arhgef5. J Biol Chem. 2009;284:28599–606.
Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–6.
Bagci H, Sriskandarajah N, Robert A, Boulais J, Elkholi IE, Tran V, et al. Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat Cell Biol. 2020;22:120–34.
Garcia-Mata R, Boulter E, Burridge K. The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504.
Zalcman G, Closson V, Camonis J, Honoré N, Rousseau-Merck MF, Tavitian A, (1996) et al. RhoGDI-3 is a new GDP dissociation inhibitor (GDI). Identification of a non-cytosolic GDI protein interacting with the small GTP-binding proteins RhoB and RhoG. The Journal of biological chemistry 271:30366–74.
Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, et al. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Current biology : CB. 2002;12:307–12.
Marignani PA, Carpenter CL. Vav2 is required for cell spreading. J Cell Biol. 2001;154:177–86.
Prisco A, Vanes L, Ruf S, Trigueros C, Tybulewicz VL. Lineage-specific requirement for the PH domain of Vav1 in the activation of CD4+ but not CD8+ T cells. Immunity. 2005;23:263–74.
Lee J, Park B, Kim G, Kim K, Pak J, Kim K, et al. Arghef16, a novel Elmo1 binding partner, promotes clearance of apoptotic cells via RhoG-dependent Rac1 activation. Biochim Biophys Acta. 2014;1843:2438–47.
Brunet N, Morin A, Olofsson B. RhoGDI-3 regulates RhoG and targets this protein to the Golgi complex through its unique N-terminal domain. Traffic (Copenhagen, Denmark). 2002;3:342–57.
Brisac C, Salloum S, Yang V, Schaefer EA, Holmes JA, Chevaliez S, et al. IQGAP2 is a novel interferon-alpha antiviral effector gene acting non-conventionally through the NF-κB pathway. J Hepatol. 2016;65:972–9.
Vigorito E, Bell S, Hebeis BJ, Reynolds H, McAdam S, Emson PC, et al. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol Cell Biol. 2004;24:719–29.
Martínez-Martín N, Fernández-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–22.
Vaeth M, Feske S. (2018) NFAT control of immune function: New Frontiers for an Abiding Trooper. F1000Research; 7:260.
Charpentier JC, King PD. Mechanisms and functions of endocytosis in T cells. Cell Commun Signal. 2021;19:92.
Prieto-Sánchez RM, Bustelo XR. Structural basis for the signaling specificity of RhoG and Rac1 GTPases. J Biol Chem. 2003;278:37916–25.
Macián F, García-Cózar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–31.
Hogan PG. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium. 2017;63:66–9.
de León-Bautista MP, Cardenas-Aguayo MD, Casique-Aguirre D, Almaraz-Salinas M, Parraguirre-Martinez S, Olivo-Diaz A, et al. Immunological and functional characterization of RhoGDI3 and Its molecular Targets RhoG and RhoB in human pancreatic cancerous and normal cells. PLoS ONE. 2016;11: e0166370.
Oh HM, Yu CR, Golestaneh N, Amadi-Obi A, Lee YS, Eseonu A, et al. STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J Biol Chem. 2011;286:30888–97.
Lämmermann T, Germain RN. The multiple faces of leukocyte interstitial migration. Seminars in immunopathology. 2014;36:227–51.
Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, et al. P-Rex1 regulates neutrophil function. Current biology : CB. 2005;15:1867–73.
Lawson CD, Donald S, Anderson KE, Patton DT, Welch HC. (2011) P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. Journal of immunology (Baltimore, Md : 1950); 186:1467–76.
Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, et al. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–21.
Pantarelli C, Welch HCE. Rac-GTPases and Rac-GEFs in neutrophil adhesion, migration and recruitment. Eur J Clin Invest. 2018;48(Suppl 2): e12939.
Condliffe AM, Webb LM, Ferguson GJ, Davidson K, Turner M, Vigorito E, et al. (2006) RhoG regulates the neutrophil NADPH oxidase. Journal of immunology (Baltimore, Md : 1950); 176:5314–20.
Mao Y, Finnemann SC. Regulation of phagocytosis by Rho GTPases. Small GTPases. 2015;6:89–99.
Tzircotis G, Braga VM, Caron E. RhoG is required for both FcγR- and CR3-mediated phagocytosis. J Cell Sci. 2011;124:2897–902.
Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–44.
Rougerie P, Miskolci V, Cox D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol Rev. 2013;256:222–39.
Kalinichenko A, Perinetti Casoni G, Dupré L, Trotta L, Huemer J, Galgano D, et al. RhoG deficiency abrogates cytotoxicity of human lymphocytes and causes hemophagocytic lymphohistiocytosis. Blood. 2021;137:2033–45.
Damoulakis G, Gambardella L, Rossman KL, Lawson CD, Anderson KE, Fukui Y, et al. P-Rex1 directly activates RhoG to regulate GPCR-driven Rac signalling and actin polarity in neutrophils. J Cell Sci. 2014;127:2589–600.
Welch HC. Regulation and function of P-Rex family Rac-GEFs. Small GTPases. 2015;6:49–70.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–93.
Yamaki N, Negishi M, Katoh H. RhoG regulates anoikis through a phosphatidylinositol 3-kinase-dependent mechanism. Exp Cell Res. 2007;313:2821–32.
Harada K, Hiramoto-Yamaki N, Negishi M, Katoh H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp Cell Res. 2011;317:1701–13.
Dipankar P, Kumar P, Sarangi PP. (2023) In silico identification and characterization of small-molecule inhibitors specific to RhoG/Rac1 signaling pathway. Journal of biomolecular structure and dynamics; 41:560–580.
Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency Blood. J Am Soc Hematology. 2000;96:1646–54.
Ahmad Mokhtar AM, Salikin NH, Haron AS, Amin-Nordin S, Hashim IF, Mohd Zaini Makhtar M, et al. (2022) RhoG’s role in T cell activation and function. Frontiers in Immunology 13:845064.
Utech M, Höbbel G, Rust S, Reinecke H, Assmann G, Walter M. Accumulation of RhoA, RhoB, RhoG, and Rac1 in fibroblasts from Tangier disease subjects suggests a regulatory role of Rho family proteins in cholesterol efflux. Biochem Biophys Res Commun. 2001;280:229–36.
Lougaris V, Baronio M, Gazzurelli L, Benvenuto A, Plebani A. RAC2 and primary human immune deficiencies. J Leucocyte Bio. 2020;108:687–96.
Weksler B, Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol. 2014;9:S137–42.
Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochemical J. 2000;348:241–55.
Roux P, Gauthier-Rouvière C, Doucet-Brutin S, Fort P. The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Curr Biol. 1997;7:629–37.
Acknowledgements
This work was supported by the Science Engineering Research Board, Govt. of India (CRG-2020-0025), to PPS; DBT fellowship to SKR. The authors would like to thank Dr. Prerna Sharma for her critical comments on the manuscript.
Author information
Authors and Affiliations
Contributions
SKR and DS have searched the literature, prepared the figures, and written the manuscript. PPS has organized and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rai, S.K., Singh, D. & Sarangi, P.P. Role of RhoG as a regulator of cellular functions: integrating insights on immune cell activation, migration, and functions. Inflamm. Res. 72, 1453–1463 (2023). https://doi.org/10.1007/s00011-023-01761-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01761-9