Abstract
The blood levels of neutrophils are associated with the severity of COVID -19. However, their role in the pulmonary environment during COVID -19 severity is not clear. Here, we found a decrease in the neutrophil count in BAL (bronchoalveolar lavage) in non-survivors and in older patients (> 60 years). In addition, we have shown that older patients have higher serum concentration of CXCL8 and increased IL-10 expression by neutrophils.
Similar content being viewed by others
Introduction
COVID-19 is an important cause of acute respiratory distress syndrome (ARDS) [1]. ARDS is a lung injury characterized by widespread inflammation, increased permeability, pulmonary edema and loss of aerated lung tissue, leading to hypoxemia and bilateral radiographic opacities [2]. ARDS is more common and severe in elderly patients and the use of invasive mechanical ventilation (IMV) is essential for life support in these critically ill patients [3].
Neutrophils play an important role in sepsis and ARDS [4], and the impaired migration of neutrophils to the site of infection may contribute to the severity of the disease [5]. Previous studies have reported neutrophilia and increased neutrophil infiltration in the lungs of patients with severe COVID-19 [4, 6]. Elderly patients show impaired neutrophil function [7] and also poorer prognosis COVID-19. It is not clear whether and how neutrophils contribute to the higher severity and mortality in elderly patients. Here, we investigated the differences in the number of neutrophils in BAL and blood between young and elderly patients with severe COVID-19 and assessed factors related to their migration and activation.
Methods
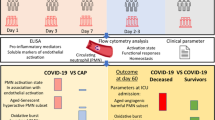
Patients with severe COVID-19, confirmed by qRT-PCR, were recruited in the Intensive Care Unit of Hospital das Clínicas of Belo Horizonte (MG). All patients or their representatives signed an informed consent (IRB approval #30437020.9.3001.5124). Patients (n = 23) were divided into four groups: Survivors (n = 13) or non-survivors (n = 10) and younger (n = 10) or older than 60 years (n = 13).
Mini broncho alveolar lavage (BAL) samples were collected in the first week after intubation at the same days of blood collection. Cells were labeled with anti-CD16, anti-CD14, anti-IL10, anti-TNF, and anti-IFN-γ and analyzed by flow cytometry. The populations of granulocytes and monocytes (SSC-A x FSC-A) and singlet (FSC-H x FSC-A) were selected. Then, the dot plot of CD16 x CD14 was used to identify the neutrophil subpopulation (CD16hiCD14int).
CXCL8 concentrations in plasma and BAL was determined using the Bio-Plex Pro™ Human Cytokine 17-plex Assay, Cat #M5000031YV (Bio-Rad).
Results
The mortality rate of patients enrolled in this study was 57% (Fig. 1A). In the survivor group, 80% were male and the median age was 56 ± 13 years old. In contrast, non-survivors were 62% male, and the median age was 70 ± 14 years (P = 0.066 compared to survivors). The frequency and number of neutrophils were reduced in the BAL of non-survivors (Fig. 1B, C). These results suggest an association between neutrophils in BAL and severity of COVID-19.
Neutrophils are reduced in the BAL of non-survivors and patients over than 60 years with severe COVID-19. Curve of survival from COVID-19 patients under mechanical ventilation (A). Flow cytometry analysis of neutrophils frequencies (B) and numbers (C) in survivor group (n = 10) and non-survivor group (n = 13). Survival curve of patients younger (< 60 years of age) and older (> 60 years of age) (D). Flow cytometry analysis of the neutrophil frequencies (E) and numbers (F). Frequencies (G) and numbers (H) of neutrophils in younger survivors (n = 7), younger non-survivors (n = 3), older survivors (n = 3), and older non-survivors (n = 10). Flow cytometry analysis of MFI of IL-10 (I), IFN-γ (J) and TNF (K) expressed by neutrophils. CXCL8 concentration (L) in the BAL of younger and older patients with COVID-19. Horizontal lines represent geometric mean ± 95% CI. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA followed by Newman–Keuls multiple comparisons post-test or by a t-test, to compare medians of the two groups. Survival analysis was made by Log Rank test. Outliers were identified using the ROUT (Q = 2.0%) method of GraphPad Prism program version 9.0 and removed from the analysis
Since older age is a risk factor for COVID -19 mortality and neutrophil dysfunction [7], we investigated whether this factor could be related to neutrophil count in BAL and blood. Patients were divided into younger (< 60-year-old) and older (> 60-year-old) groups. In the older group, 62% were male and the mean age was 74 ± 8 years, significantly higher than in the younger group, 49 ± 8 years (P < 0.001) and 80% of male. The older group had higher mortality than the younger group (80% versus 25%, P < 0.05) (Fig. 1D). The number and frequency of neutrophils were reduced in the BAL of older patients (Fig. 1E, F). However, in blood, the frequency of neutrophils, but not their absolute number, was higher in the older group than in the younger group (Table 1). Interestingly, the younger patients who died of COVID-19 also had a lower frequency of neutrophils (Fig. 1G), but not in absolute numbers (Fig. 1H), in BAL compared to survivor younger patients.
To determine the activation status of neutrophils, we examined the expression of cytokines. We found that IL-10 expression by BAL neutrophils was higher in the older group than in younger group (Fig. 1I), but no difference was observed in IFN- γ or TNF expression (Fig. 1J, K). No difference was observed in cytokine expression by blood neutrophils between the groups (Table 1).
Because our data indicated a disturbance of neutrophil migration in the older group (high neutrophil levels in blood and low levels in BAL), we examined CXCL8, a chemokine that regulates neutrophil traffic. BAL CXCL8 levels were similar in the older and the younger patients (Fig. 1L), but older patients had higher blood CXCL8 concentrations when compared to younger patients (Table 1).
Discussion
Severe COVID-19 is characterized by changes in the profile of circulating neutrophils, with higher neutrophil/lymphocyte rate [4]. Several studies have reported an increase in neutrophil infiltrate in the lungs of severe COVID-19 patients, but it is not clear whether neutrophils are related to disease severity [8, 9]. We found that mortality due to COVID-19 was associated with decreased neutrophil count in the lungs, especially in older patients (> 60-year-old). Interestingly, neutrophils from older patients produced increased levels of IL-10, which may regulate the inflammatory response [10]. Coherently, studies have also shown increased PD-L1 surface expression [11] and downregulation of CD62L in neutrophils from severe COVID-19 patients, suggesting a suppressive phenotype [12]. In addition, we found higher concentrations of CXCL8 in the blood of older patients, but not in BAL, which may lead to changes in the profile and impaired migratory capacity to the lungs of neutrophils due to desensitization of CXCR2 [5].
In conclusion, our data show that a reduction in the number of neutrophils in BAL is associated with worse prognosis in severe COVID-19 elderly patients.
Data availability statement
The data that support the findings of this study are available from the corresponding author DGS request.
References
World Heath Organization . WHO Coronavirus (COVID-19) Dashboard. 2023. Available Online:https://covid19.who.int. Accessed 18 Mar 2023.
Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20(5):270–84.
Grasselli G, Cattaneo E. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care. 2021;25(1):115.
Fu J, Kong J. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3–8.
Sonego F, Castanheira FV. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front Immunol. 2016;7:155.
Goncalves-Pereira MH, Santiago L. Dysfunctional phenotype of systemic and pulmonary regulatory T cells associate with lethal COVID-19 cases. Immunology. 2022;2:1.
Bartleson JM, Radenkovic D. SARS-CoV-2, COVID-19 and the ageing immune system. Nat Aging. 2021;1(9):769–82.
Liew PX, Kubes P. The Neutrophil’s role during health and disease. Physiol Rev. 2019;99(2):1223–48.
Reusch N, De Domenico E. Neutrophils in COVID-19. Front Immunol. 2021;12: 652470.
Ocuin LM, Bamboat ZM. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol. 2011;89(3):423–32.
MacDonald L, Alivernini S. COVID-19 and RA share an SPP1 myeloid pathway that drives PD-L1+ neutrophils and CD14+ monocytes. JCI Insight. 2021;6:13.
Schulte-Schrepping J, Reusch N. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182(6):1419–40.
Acknowledgements
We are grateful to study participants, and the Institutional Laboratory of Biomarkers Research (LINBIO) for support with the flow cytometry.
Funding
This work was supported by SESU-MEC (public notice “Fighting COVID-19”), INCT-Vacinas, CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior) and INCT-dengue e Interação Microrganismo hospedeiro.
Author information
Authors and Affiliations
Contributions
MHG-P, LS, CGR, PF V, VN and HCS conceived the study design. LS, CGR and PFV recruited study participants and collected biological samples. MHG-P performed cell culture and flow cytometry staining. FFSO and APS provided support with the flow cytometry platform. RDNA, MHG-P, MMT, HCS and DGS analyzed and interpreted data. RDNA, MHG-P, MMT, HCS and DGS prepared the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare they have no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arifa, R.D.N., Gonçalves-Pereira, M.H., Santiago, L. et al. Reduction in the number of neutrophils in the broncho-alveolar aspirate is associated with worse prognosis in elderly patients with severe COVID-19. Inflamm. Res. 72, 929–932 (2023). https://doi.org/10.1007/s00011-023-01726-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01726-y