Abstract
Objective
Cytokine storm syndrome is a fatal condition related to infectious and autoimmune diseases. Here, we aim to investigate the regulatory mechanisms of Blimp-1 on multiple cytokine production.
Methods
The Blimp1 shRNA was transfected into RAW264.7 macrophages, followed by Toll-like receptor (TLR) ligand stimulation. The mRNA and protein levels of cytokines were detected by real-time PCR and flow cytometric bead array. The nuclear translocation of AP-1 and NF-κB p65 was measured by immunofluorescence staining. The transcriptional activity was detected by luciferase reporter assay with 5 × NF-κB reporter or with IL6 promoter reporter.
Results
Blimp-1 significantly inhibited the expression and secretion of IL-1β, IL-6, and IL-18 in macrophages during stimulation with a variety of TLR ligands. The immunofluorescence staining results showed that Blimp-1 strictly controlled the nuclear translocation of NF-κB p65 in LPS-challenged macrophages. Furthermore, Blimp-1 directly inhibited the transcriptional activity of NF-κB and the transcription of IL6 gene.
Conclusion
Blimp-1 represses the production of multiple pro-inflammatory cytokines by directly binding the genomic region and restricting the nuclear translocation and transcriptional activity of NF-κB. This finding may provide potential therapeutic strategies for the cytokine storm-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytokine storm syndrome is a fatal condition that occurs when the immune system responds aggressively in infectious diseases, autoimmune diseases, and CAR T-cell therapy. Cytokine storm plays a key regulatory role in sepsis, unfortunately, so far, most clinical trials simultaneously targeting multiple cytokine production or effect failed [1]. Drugs blocking TNF-α and IL-6 have been approved for the treatment of rheumatoid arthritis, but the blockade of a single cytokine has a limited therapeutic effect on the disease [2]. Therefore, therapies targeting multiple pro-inflammatory cytokines may provide new ideas for the treatment of cytokine storm-related diseases.
Transcription factor B lymphocyte-induced maturation protein (Blimp-1) is a key regulator of plasma cell differentiation in B cells and effector/memory function in T cells [3, 4]. Blimp-1 is also required to maintain immune tolerance in myeloid cells and embryonic intestine [5, 6]. For example, conditional deletion of Blimp-1 in dendritic cells (DCs) showed an increased production of IL-6 and resulted in aberrant activation of the adaptive immune system in systemic lupus erythematous [7]. As mentioned above, Blimp-1 acts as a “Brake pad” in regulating immune functions; however, the regulatory mechanism of Blimp-1 in myeloid cells remains unclear. Blimp-1 is a SET domain and zinc finger-containing transcriptional repressor. Our previous study has demonstrated that it directly binds to the promoters of PD-1 and TIGIT in T cells [8]. In the present study, we aim to reveal the potential roles and mechanisms of Blimp-1 in regulating multiple cytokine production in macrophages. The results may provide new insight into the mechanisms of cytokine storm formation and potential therapeutic targets.
Materials and methods
See supplementary material.
Results
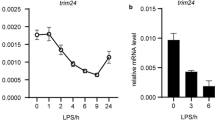
To elucidate the regulation of Blimp-1 on the pro-inflammatory cytokine production, we stably transfected RAW264.7 macrophages with Blimp1 shRNA followed by stimulation with different TLR ligands to mimic invasive pathogens (Fig. S1). We stimulated the cells with Pam3CSK4 (TLR2 ligand), PolyI:C (TLR3 ligand), Lipopolysaccharide (LPS; TLR4 ligand), and CpG-ODN (TLR9 ligand) for 3, 6, 12, and 24 h, the secretion of cytokine IL-1β, IL-6, and IL-18 was significantly elevated by interfering with Blimp-1 (Fig. 1a). Consistently, by knocking-down Blimp-1, the mRNA levels of Il1β, Il6, and Il18 were increased as early as 3 h after TLR ligand stimulations (Fig. 1b). Exceptionally, the expression and secretion of Tnf in Blimp1 shRNA transfection was comparable to that in the non-specific control shRNA (Fig. 1a, b). By analyzing the Blimp-1 ChIP-seq data from a previous study [9], we observed that Blimp-1 had stable binding at both the proximal promoters of Il1b and Tnf, and the distal enhancers of Il6 and Il18 (Fig. S2). These results indicate that Blimp-1 has a strong inhibitory effect on a variety of pro-inflammatory cytokines at the transcription level during different infections.
The effect of Blimp-1 on the production of pro-inflammatory cytokines and on the transcriptional activity of NF-κB. a The cells were transfected with Blimp-1 shRNA or non-specific control and then stimulated with Pam3CSK4, PolyI:C, LPS, and CpG-ODN for 3, 6, 12 and 24 h. The contents of IL-1β, IL6, IL18, and TNF-α in the culture medium were detected by flow cytometric bead array analysis. b The mRNA levels of Il1b, Il6, Il18, and Tnf in RAW264.7 cells. c Immunofluorescence staining of AP-1 (red), NF-κB p65 (red), and DAPI (blue) in RAW 264.7 cells. The mean fluorescent intensity (MFI) of AP-1 and NF-κB p65 in nuclear was quantified and compared between groups. Scale bar = 20 μm. ***, p < 0.001; **, p < 0.01; *, p < 0.05. d Reporter gene assay was performed with 293 T cells transfected with a Gal4-p65 expressing plasmid and a reporter construct containing five copies of the NF-κB-binding site (5 × NF-κB), with either pcDNA3 or the Blimp-1 (PRDM1b) expressing plasmid. e Dual luciferase assay were performed with 293 T cells transfected with Blimp-1 (PRDM1b) expressing plasmid or the vehicle, as well as three reporters of IL6 promoter, wild type (WT), NF-κB-binding site mutation (mNF-κB), and AP-1-binding site mutation (mAP-1), respectively. RLA, relative luciferase activity
TLRs activate the transcription factor NF-κB and activator protein-1 (AP-1), which consequently induces inflammatory responses. A hallmark activation of NF-κB and AP-1 is nuclear translocation, which is necessary for modulating gene transcription. By stimulating RAW264.7 cells with LPS, we found that Blimp-1 knockdown significantly up-regulated the expressions of p65 and AP-1 (Fig. 1c). Especially after Blimp-1 was knocked out, it was remarkably increased in the cytoplasmic to nuclear translocation of p65 (Fig. 1c). These data suggest that Blimp-1 inhibits the transcription of multiple pro-inflammatory cytokines by restricting the entry of NF-κB p65 into the nucleus.
Moreover, we used a reporter construct containing five copies of the NF-κB-binding site (5 × NF-κB) to show an inhibitory effect of Blimp-1 on the transcriptional activity of NF-κB (Fig. 1d). We further constructed three reporters of IL6 promoter, wildtype (WT), NF-κB-binding site mutation (mNF-κB), and AP-1-binding site mutation (mAP-1), and performed dual luciferase reporter assays. We found that overexpression of Blimp-1 significantly inhibited the activities of WT and mAP-1 IL6 promoter; however, no distinct inhibitory effect was observed on the mNF-κB IL6 promoter (Fig. 1e). These data provide direct evidences that Blimp-1 controls the transcriptional activity and promoter binding of NF-κB.
Discussion
Here, we demonstrated the inhibitory effect of Blimp-1 in pro-inflammatory cytokine production in macrophages. Blimp-1 has been reported to regulate myeloid cell terminal differentiation. Blimp-1 acts as a regulation of the macrophages pyroptosis in response to L. donovani infection [10]. Our findings further revealed the function and mechanism of Blimp-1 in maintaining appropriate inflammation in macrophages against infections. Since macrophages possess immunomodulatory and inflammatory abilities and actively participate in the development of many diseases, Blimp-1 may play a broad role in inflammatory diseases in which macrophages are involved.
We found that Blimp-1 directly binds to promoters or enhancers of IL-1β, IL-6, and IL-18 coding genes and inhibits nuclear translocation of NF-κB p65, thereby reducing the cytokine secretion. Notably, although Blimp-1 binds to the Tnf promoter, it does not inhibit the expression and secretion of TNF-α. There are two possible explanations. First, transcriptional repression by Blimp-1 involves recruitment of histone deacetylase, and the inability to regulate Tnf transcription may be due to dysregulation of histone deacetylation [11]. Second, there may be other transcription factors that act antagonistically to regulate Tnf transcription. Therefore, the transcriptional regulation mechanism of TNF-α still needs to be further elucidated.
In conclusion, Blimp-1 tightly controls the expression and secretion of multiple pro-inflammatory cytokines, which may be the key hub for regulating the inflammatory response. Although it is still necessary to further demonstrate the pathophysiological role of Blimp-1, this study provides new ideas and potential therapeutic strategies for the cytokine storm-related diseases.
Data availability statement
All data generated or analyzed during this study are included in this article and its supplementary information file.
References
Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73.
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97.
Sledzinska A, Vila de Mucha M, Bergerhoff K, Hotblack A, Demane DF, Ghorani E, et al. Regulatory T cells restrain interleukin-2- and blimp-1-dependent acquisition of cytotoxic function by CD4(+) T cells. Immunity. 2020;52:151-166 e6.
Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–20.
Nadeau S, Martins GA. Conserved and unique functions of blimp1 in immune cells. Front Immunol. 2021;12: 805260.
Ulmert I, Henriques-Oliveira L, Pereira CF, Lahl K. Mononuclear phagocyte regulation by the transcription factor Blimp-1 in health and disease. Immunology. 2020;161:303–13.
Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. J Exp Med. 2011;208:2193–9.
Zhu L, Kong Y, Zhang J, Claxton DF, Ehmann WC, Rybka WB, et al. Blimp-1 impairs T cell function via upregulation of TIGIT and PD-1 in patients with acute myeloid leukemia. J Hematol Oncol. 2017;10:124.
Shin HM, Kapoor V, Guan T, Kaech SM, Welsh RM, Berg LJ. Epigenetic modifications induced by Blimp-1 regulate CD8(+) T cell memory progression during acute virus infection. Immunity. 2013;39:661–75.
Saha G, Khamar BM, Prerna K, Kumar M, Dubey VK. BLIMP-1 plays important role in the regulation of macrophage pyroptosis for the growth and multiplication of Leishmania donovani. ACS Infect Dis. 2019;5:2087–95.
Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol. 2000;20:2592–603.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81671940, 81871586, and 82172128) and Beijing Municipal Excellent Talents Foundation (2018000021223ZK27).
Author information
Authors and Affiliations
Contributions
QQ and RL performed the experiments, analyzed the data and did the statistical analysis. LL performed the experiments. LL, YZ, and SD participated in performing the experiments. QQ and LZ wrote the paper. LZ designed the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, Q., Li, R., Li, L. et al. Multi-target regulation of pro-inflammatory cytokine production by transcription factor Blimp-1. Inflamm. Res. 72, 217–220 (2023). https://doi.org/10.1007/s00011-022-01671-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01671-2