Abstract
Objective
To investigate the participation of canonical Wnt and NF-κB signaling pathways in an experimental model of chronic arthritis induced by methylated bovine serum albumin (mBSA) in rat temporomandibular joint (TMJ).
Materials and methods
Wistar rats were sensitized by mBSA+Complete Freund Adjuvant (CFA)/Incomplete Freund Adjuvant (IFA) on the first 14 days (1 ×/week). Subsequently, they received 1, 2 or 3 mBSA or saline solution injections into the TMJ (1 ×/week). Hypernociceptive threshold was assessed during the whole experimental period. 24 h after the mBSA injections, the TMJs were removed for histopathological and immunohistochemical analyses for TNF-α, IL-1β, NF-κB, RANKL, Wnt-10b, β-catenin and DKK1.
Results
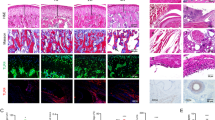
The nociceptive threshold was significantly reduced after mBSA injections. An inflammatory infiltrate and thickening of the synovial membrane were observed only after mBSA booster injections. Immunolabeling of TNF-α, IL-1β and Wnt-10b was increased in the synovial membrane in arthritic groups. The immunoexpression of nuclear β-catenin was significantly higher only in the group that received 2 booster TMJ injections. However, NF-κB, RANKL and DKK1 immunoexpression were increased only in animals with 3 mBSA intra-articular injections.
Conclusion
Our results suggest that canonical Wnt and NF-κB signaling pathways participate in the hypernociception and inflammatory response in TMJ synovial membrane during the development of rheumatoid arthritis in rats.






Similar content being viewed by others
References
Cunha CO, Pinto LMS, Mendonça LM, Saldanha ADD, Conti ACCF, Conti PCR. Bilateral asymptomatic fibrous-ankylosis of the temporomandibular joint associated with rheumatoid arthritis: a case report. Braz Dent J. 2012;23:77982. https://doi.org/10.1590/s0103-64402012000600025.
Sodhi A, Naik S, Pai A, Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent. 2015;6:124–7. https://doi.org/10.4103/0976-237X.149308.
Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. https://doi.org/10.1186/ar2669.
Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y, Zhang L, Lv XW, Jin Y, Li J. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25:2069–78. https://doi.org/10.1016/j.cellsig.2013.04.002.
Ruparelia PB, Shah DS, Ruparelia K, Sutaria SP, Pathak D. Bilateral TMJ involvement in rheumatoid arthritis. Case Rep Dent. 2014;2014:262430. https://doi.org/10.1155/2014/262430.
Ahmed N, Catrina AI, Alyamani AO, Mustafa H, Alstergren P. Deficient cytokine control modulates temporomandibular joint pain in rheumatoid arthritis. Eur J Oral Sci. 2015;123:235–41. https://doi.org/10.1111/eos.12193.
Ke J, Long X, Liu Y, Zhang YF, Li J, Fang W, Meng QG. Role of NF-κB, in TNF-α induced COX-2 expression in synovial fibroblasts from human TMJ. J Dent Res. 2007;86:363–7. https://doi.org/10.1177/154405910708600412.
Quinteiro MS, Napimoga MH, Macedo CG, Freitas FF, Abdalla HB, Bonfante R, Trindade-Clemente Napimoga J. 15-deoxy-Δ12,14-prostaglandin J2 reduces albumin-induced arthritis in temporomandibular joint of rats. Eur J Pharmacol. 2014;740:58–65. https://doi.org/10.1016/j.ejphar.2014.07.002.
Chandrupatla DMSH, Weijers K, Gent YYJ, Greeuw I, Lammertsma AA, Jansen G, Laken CJ, Molthoff CFM. Sustained macrophage infiltration upon multiple intra-articular injections: an improved rat model of rheumatoid arthritis for PET guided therapy evaluation. Biomed Res Int. 2015;2015:509295. https://doi.org/10.1155/2015/509295.
Bonfante R, Napimoga MH, Macedo CG, Abdalla HB, Pieroni V, Clemente Napimoga JT. The P2X7 receptor, cathepsin S and fractalkine in the trigeminal subnucleus caudalis signal persistent hypernociception in temporomandibular rat joints. Neuroscience. 2018;391:120–30. https://doi.org/10.1016/j.neuroscience.2018.09.005.
Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–16. https://doi.org/10.1002/art.22321.
Imai K, Morikawa M, D’Armiento J, Matsumoto H, Komiya K, Okada Y. Differential expression of WNTs and FRPs in the synovium of rheumatoid arthritis and osteoarthritis. Biochem Biophys Res Commun. 2006;345:1615–20. https://doi.org/10.1016/j.bbrc.2006.05.075.
Gatica-Andrades M, Vagenas D, Kling J, Nguyen TTK, Benham H, Thomas R, Körner H, Venkatesh B, Cohen J, Blumenthal A. WNT ligands contribute to the immune response during septic shock and amplify endotoxemia-driven inflammation in mice. Blood Adv. 2017;1:1274–86. https://doi.org/10.1182/bloodadvances.2017006163.
Humphries AC, Mlodzik M. From instruction to output: Wnt/PCP signalling in development and cancer. Curr Opin Cell Biol. 2017;51:110–6. https://doi.org/10.1016/j.ceb.2017.12.005.
Sen M. Wnt signaling in rheumatoid arthritis. Rheumatology. 2005;44:708–13. https://doi.org/10.1093/rheumatology/keh553.
Liu YR, Yan X, Yu HX, Yao Y, Wang JQ, Li XF, Chen RN, Xu QQ, Ma TT, Huang C, Li J. NLRC5 promotes cell proliferation via regulating the NF-κB signaling pathway in rheumatoid arthritis. Mol Immunol. 2017;91:24–34. https://doi.org/10.1016/j.molimm.2017.08.024.
Xia ZB, Meng FR, Fang YX, Wu X, Zhang CW, Liu Y, Liu D, Li GQ, Feng FB, Qiu HY. Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis. Medicine. 2018;97:e10920. https://doi.org/10.1097/md.0000000000010920.
Rigoglou S, Papavassiliou AG. The NF-κB signaling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–4. https://doi.org/10.1016/j.biocel.2013.08.018.
Chandrakesan P, Jakkula LU, Ahmed I, Roy B, Anant S, Umar S. Differential effects of β-catenin and NF-κB interplay in the regulation of cell proliferation, inflammation and tumorigenesis in response to bacterial infection. PloS One. 2013;8:e79432. https://doi.org/10.1371/journal.pone.0079432.
Lora VRMM, Clemente-Napimoga JT, Abdalla HB, Macedo CG, Canales GT, Barbosa CMR. Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomandibular joint of rats. Toxicon. 2017;129:52–7. https://doi.org/10.1016/j.toxicon.2017.02.010.
Gondim DV, Costa JL, Rocha SS, Brito GA, Ribeiro RA, Vale ML. Antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Can J Physiol Pharmacol. 2012;90:395–405. https://doi.org/10.1139/y2012-003.
Chaves HV, do Val DR, Ribeiro KA, Lemos JC, Souza RB, Gomes FIF, da Cunha RMS, de Paulo Teixeira Pinto V, Filho GC, de Souza MHLP, Bezerra MM, de Castro Brito GA. Heme oxygenase-1/biliverdin/carbon monoxide pathway downregulates hypernociception in rats by a mechanism dependent on cGMP/ATP-sensitive K+ channels. Inflamm Res. 2018;67:407–22. https://doi.org/10.1007/s00011-018-1133-z.
Liu YD, Liao LF, Zhang HY, Lu L, Jiao K, Zhang M, Wang MQ. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage. 2014;22:302–12. https://doi.org/10.1016/j.joca.2013.11.014.
Oliveira MC, Tavares LP, Vago JP, Batista NT, Queiroz-Junior CM, Vieira AT, Ferreira AVM. Tumor Necrosis Factor, but not neutrophils, alters the metabolic profile in acute experimental arthritis. Plos One. 2016;11:e0146403. https://doi.org/10.1371/journal.pone.0146403.
Farinon M, Lora PS, Francescato LN, Bassani VL, Henriques AT, Xavier RM, De Oliveira PG. Effect of Aqueous Extract of Giant Horsetail (Equisetum giganteum L.) in Antigen-Induced Arthritis. Open Rheumatol J. 2013;7:129–33. https://doi.org/10.2174/1874312901307010129.
Westacott CI, Witcher JT, Barnes IC, Thompson D, Swan AJ, Dieppe PA. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990;49:676–81. https://doi.org/10.1136/ard.49.9.676.
Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52:870–5. https://doi.org/10.1136/ard.52.12.870.
Towle CE, Hung HH, Bonassar LJ, Treadwell BV. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5:293–300. https://doi.org/10.1016/s1063-4584(97)80008-8.
Fassio A, Adami G, Gatti D, Orsolini G, Giollo A, Idolazzi L, Benini C, Vantaggiato E, Rossini M, Viapiana O. Inhibition of tumor necrosis factor-alpha (TNF-alpha) in patients with early rheumatoid arthritis results in acute changes of bone modulators. Int Immunopharmacol. 2019;67:487–9. https://doi.org/10.1016/j.intimp.2018.12.050.
Raychaudhuri SP, Raychaudhuri SK. Biologics: target-specific treatment of systemic and cutaneous autoimmune diseases. Indian J Dermatol. 2009;54:100–9. https://doi.org/10.4103/0019-5154.53175.
Gertel S, Mahagna H, Karmon G, Watad A, Amital H. Tofacitinib attenuates arthritis manifestations and reduces the pathogenic CD4 T cells in adjuvant arthritis rat. Clin Immunol. 2017;184:77–81. https://doi.org/10.1016/j.clim.2017.04.015.
McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol. 2016;12:63–8. https://doi.org/10.1038/nrrheum.2015.171.
Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–8. https://doi.org/10.1038/319516a0.
Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. https://doi.org/10.1101/cshperspect.a001651.
Kim HJ, Park C, Kim GY, Park EK, Jeon YJ, Kim S, Hwang HJ, Choi YH. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-κB and activation of the Nrf2/HO-1 signaling pathway. Biosci Trends. 2018;12(3):257–65. https://doi.org/10.5582/bst.2018.01107.
Kim JM, Lee JH, Lee GS, Noh EM, Song HK, Gu DR, Kim SC, Lee SH, Kwon KB, Lee YR. Sophorae Flos extract inhibits RANKL-induced osteoclast differentiation by suppressing the NF-κB/NFATc1 pathway in mouse bone marrow cells. BMC Complement Altern Med. 2017;17(1):164. https://doi.org/10.1186/s12906-016-1550-x.
Lee EJ, So MW, Hong S, Kim YG, Yoo B, Lee CK. Interleukin-33 acts as a transcriptional repressor and extracellular cytokine in fibroblast-like synoviocytes in patients with rheumatoid arthritis. Cytokine. 2016;77:35–43. https://doi.org/10.1016/j.cyto.2015.10.005.
Boman A, Kokkonen H, Arlestig L, Berglin E, Rantapãã-Dahlqvist S. Receptor activator of nuclear factor kappa-B ligand (RANKL) but not sclerotin or gene polymorphisms is related to joint destruction in early rheumatoid arthritis. Clin Rheumatol. 2017;2017(36):1005–12. https://doi.org/10.1007/s10067-017-3570-4.
Rabelo FS, da Mota LM, Lima RA, Lima FA, Barra GB, de Carvalho JF, Amato AA. The Wnt signaling pathway and rheumatoid arthritis. Autoim Rev. 2019;9:207–10. https://doi.org/10.1016/j.autrev.2009.08.003.
Xiao CY, Pan YF, Guo XH, Wu YQ, Gu JR, Cai DZ. Expression of β-catenin in rheumatoid arthritis fibroblast-like synoviocytes. Scand J Rheumatol. 2011;2011(40):26–33. https://doi.org/10.3109/03009742.2010.486767.
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. https://doi.org/10.1056/NEJM200103223441207.
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;2003(423):356–61. https://doi.org/10.1038/nature01661.
Van der Bosh MH, Blom AB, Sloetjes AW, Koenders MI, van de Loo FA, van den Berg WB, van Lent PL, van der Kraam PM. Induction of canonical Wnt signaling by synovial overexpression of selected Wnts leads to protease activity and early osteoarthritis-like cartilage damage. Am J Pathol. 2015;185(7):1970–80. https://doi.org/10.1016/j.ajpath.2015.03.013.
Van der Bosh MH, Blom AB, van de Loo FA, Koenders MI, Lafeber FP, van den Berg WB, van der Kraam PM, van Lent PL. Synovial Wnt signaling induces the expression. Of MMPs and is associated with disease progression in early symptomatic osteoarthritis patients. Arthritis Rheumatol. 2017;69(10):1978–83. https://doi.org/10.1002/art.40206.
Chu CQ, Field M, Feldmann M, Maini RA. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage- pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–32. https://doi.org/10.1002/art.1780340908.
Kraan MC, Patel DD, Haringman JJ, Smith MD, Weedon H, Ahern MJ, Breedveld FC, Tak PP. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synthesis of the chemokine CXCL8 (interleukin-8). Arthritis Res. 2002;3(1):65–71. https://doi.org/10.1186/ar141.
Malysheva K, Rooji K, Lowik CWGM, Baten DL, Rose-John S, Stoika R, Korchynskyi O. Interleukin6/Wnt interactions in rheumatoid arthritis: interleukin 6 inhibits Wnt signaling in synovial fibroblasts and osteoblast. Croat Med J. 2016;57(2):89–98. https://doi.org/10.3325/cmj.2016.57.89.
Honsawek S, Tanavelee A, Yuktanandana P, Ngarmukos S, Saetan N, Tantavisut S. Dickkopf-1 (DKK-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskel Dis. 2010;11:257. https://doi.org/10.1186/1471-2474-11-257.
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the b-catenin/TCF pathway. Oncogene. 2004;23:8520–6. https://doi.org/10.1038/sj.onc.1207892.
Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24(1):73–84. https://doi.org/10.1038/sj.emboj.7600460.
Ma B, Fey M, Hottiger MO. Wnt/β-catenin signaling inhibits CBP- mediated RelA acetylation and expression. Of proinflammatory NF-κB target genes. J Cell Sci. 2015;128:2430–6. https://doi.org/10.1242/jcs.168542.
Ma B, Hottiger MO. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front Immunol. 2016;7:378. https://doi.org/10.3389/fimmu.2016.00378.
Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatology. 2013;57:763–74. https://doi.org/10.1002/hep.26042.
Yun K, Choi YD, Nam JH, Park Z, Im SH. NF-kappaB regulates Lef1 gene expression in chondrocytes. Biochem Biophys Res Commun. 2007;357:589–95. https://doi.org/10.1016/j.bbrc.2007.03.170.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Howard Lopes Ribeiro Júnior, Flávia de Araújo Silva and Adalberto Nascimento de Lima Júnior. This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa, L.M., dos Santos Alves, J.M., da Silva Martins, C. et al. Immunoexpression of canonical Wnt and NF-κB signaling pathways in the temporomandibular joint of arthritic rats. Inflamm. Res. 68, 889–900 (2019). https://doi.org/10.1007/s00011-019-01274-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01274-4