Abstract
Objectives
Impact of ROS in development of hyperalgesia has recently motivated scientists to focus on ROS as novel target of anti-hyperalgesic interventions. However, role of ROS in molecular signaling of hyperalgesia is still poorly understood. The present study is aimed to analyze the effect of dietary antioxidant resveratrol on antioxidant defense system, ROS level and TNFR1–ERK signaling pathway during early and late phase of inflammatory hyperalgesia.
Methods and materials
Hyperalgesia was assessed by paw withdrawal latency test in complete Freund’s adjuvant-induced hyperalgesic rats. Activities of antioxidant enzymes were measured by in-gel assays, ROS level was measured by DCFH2DA, and expression of pERK, ERK and TNFR1 was estimated by Western blotting.
Results
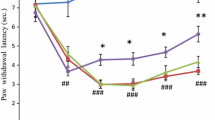
Anti-hyperalgesic effect of resveratrol was observed by paw withdrawal latency test. ROS level was increased in paw skin as well as spinal cord during early phase which was further increased in paw skin, but remained constant in spinal cord up to late phase. Resveratrol differentially regulated the activities of SOD, catalase and GPx in paw skin as well as spinal cord of hyperalgesic rats in both phases. Activities were normalized back showing anti-hyperalgesic effect of resveratrol. Upregulated ERK signaling was modulated by resveratrol, whereas TNFR1 level remained unchanged.
Conclusion
Overall results suggest that resveratrol alleviates inflammatory hyperalgesia by downregulation of ERK activation, modulation of ROS and differential regulation of antioxidant enzymes during early and late phases.






Similar content being viewed by others
References
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
Pham-Marcou TA, Beloeil H, Sun X, Gentili M, Yaici D, Benoit G, Benhamou D, Mazoit JX. Anti-nociceptive effect of resveratrol in carrageenan-evoked hyperalgesia in rats: prolonged effect related to COX-2 expression impairment. Pain. 2008;140:274–83.
Torres-Lopez JE, Ortiz MI, Castaneda-Hernandez G, Alonso-Lopez R, Asomoza-Espinosa R, Granados-Soto V. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci. 2002;70:1669–76.
Singh AK, Vinayak M. Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia via differential regulation of pro-inflammatory mediators. Phytother Res. 2016;30(7):1164–71.
Gupta YK, Sharma M, Briyal S. Antinociceptive effect of trans-resveratrol in rats: involvement of an opioidergic mechanism. Methods Find Exp Clin Pharmacol. 2004;26:667–72.
Martínez-Revelles S, Avendaño MS, García-Redondo AB, Alvarez Y, Aguado A, Pérez-Girón JV, García-Redondo L, Esteban V, Redondo JM, Alonso MJ, Briones AM, Salaices M. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal. 2013;18(1):51–65.
Doyle T, Bryant L, Muscoli C, Cuzzocrea S, Esposito E, Chen Z, Salvemini D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett. 2010;483(2):85–9.
Wartenberg M, Schallenberg M, Hescheler J, Sauer H. Reactive oxygen species-mediated regulation of eNOS and iNOS expression in multicellular prostate tumor spheroids. Int J Cancer. 2003;104(3):274–82.
Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–9.
Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–24.
Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–11.
Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17.
Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–78.
Singh AK, Vinayak M. Curcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-α, IL-1β and IL-6. Neurochem Res. 2015;40:463–72.
Harris ED. Regulation of antioxidant enzyes. FASEB J. 1992;06:2675–83.
Shull S, Henitz NH, Periasamy M, Manohar M, Janssen YMW, Marsh JP, Mossman BT. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991;266(36):24398–403.
Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87(4):1595–9.
Mills GC. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative damage. J Biol Chem. 1957;229(1):189–97.
Rittner HL, Machelska H, Stein C. Leuckocyte in the regulation of pain and analgesia. J Leukoc Biol. 2005;78:1215–22.
Hensellek S, Brell P, Schaible H, Bräuer R, Segond von Banchet G. The cytokine TNF-α increases the proportion of DRG neurons expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36:381–91.
Ma F, Zhang L, Westlund KN. Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Mol Pain. 2009;5:31.
Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48.
Ji RR, Gereau RW, Malcangioc M, Strichartza GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48.
Das L, Vinayak M. Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One. 2014;9(6):e99583.
Woodbury W, Spencer AK, Stahman MA. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971;44:301–5.
Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87.
Lin CL, Chen HJ, Hou WC. Activity staining of glutathione peroxidase after electrophoresis on native and sodium dodecylsulfate polyacrylamide gels. Electrophoresis. 2002;23:513–6.
Ren K, Dubner R. Inflammatory models of pain and hyperalgesia. ILAR J. 1999;40(3):111–8.
Gao YJ, Ji RR. c-Fos or pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–7.
Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–8.
Naik AK, Tandan SK, Dudhgaonkar SP, Jadhav SH, Kataria M, Prakash VR, Kumar D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-l-cysteine in rats. Eur J Pain. 2006;10:573–9.
Guedes RP, Bosco LD, Teixeira CM, Araújo ASR, Llesuy S, Belló-Klein A, Ribeiro MF, Partata WA. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–9.
Varija D, Kumar KP, Reddy KP, Reddy VK. Prolonged constriction of sciatic nerve affecting oxidative stressors and antioxidant enzymes in rat. Indian J Med Res. 2008;129:587–92.
Mittal N, Joshi R, Hota D, Chakrabarti A. Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother Res. 2009;23:507–12.
Stefanson AL, Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients. 2014;6:3777–801.
Noack H, Lindenau J, Rothe F, Asayama K, Wolf G. Differential expression of superoxide dismutase isoforms in neuronal and glial compartments in the course of excitotoxically mediated neurodegeneration: relation to oxidative and nitrergic stress. Glia. 1998;23(4):285–97.
Das L, Vinayak M. Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-kappaB signalling in lymphoma-bearing mice. Biosci Rep. 2012;32(2):161–70.
Kaynar MY, Hanci M, Kuday C, Belce A, Gumustas K, Kokoglu E. Changes in the activity of antioxidant enzymes (SOD, GPX, CAT) after experimental spinal cord injury. Tokushima J Exp Med. 1994;41(3–4):133–6.
Silva-Adaya D, Gonsebatt ME, Guevara J. Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev. 2014. doi:10.1155/2014/590808.
Soriano FX, Léveillé F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107(2):533–43.
Lee K, Esselman WJ. Inhibition of PTPs by H2O2 regulates the activation of distinct MAPK pathways. Free Radic Biol Med. 2002;33(8):1121–32.
Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309(4):1017–26.
Acknowledgements
Authors are thankful to DRDO, India, for financial support (Grant No. ERIP/ER/1003851/M/01/1336). AKS thanks UGC, India, for JRF and SRF. Financial support by DST-FIST and UGC-CAS program to Department of Zoology, BHU, and UGC-UPE to BHU is also acknowledged.
Author information
Authors and Affiliations
Contributions
AKS has executed the experiments. MV and AKS have contributed in conception of the study, design of experiments, analysis and interpretation of data and finalizing the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A.K., Vinayak, M. Resveratrol alleviates inflammatory hyperalgesia by modulation of reactive oxygen species (ROS), antioxidant enzymes and ERK activation. Inflamm. Res. 66, 911–921 (2017). https://doi.org/10.1007/s00011-017-1072-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1072-0