Abstract
A practical and representative sampling method for microbiological examination of the slaughter process is useful for identifying abattoir-specific risk factors within the pig slaughter line. The aim of this study was to examine the suitability of an agar contact method (ACM), where the agar was homogenized before the microbiological processing, in comparison with the wet-dry double swabbing method (WDSM) for quantitative determination of total viable counts (TVC) on pig skin surfaces. In our experimental trial, pig skin pieces were artificially contaminated at 2 levels (3 log and 7 log cfu/ml) with a suspension of bacteria species commonly found on pig skin and cultivated in vitro. Within our field trial, pig carcasses were investigated at pre-chilling in an abattoir under standard processing conditions. For both sampling methods, TVC was determined, and statistical equivalence tests were calculated. Linear regression models showed the similarity of the sampling methods, with coefficient of determination (R2) > 90% and slope parameters of nearly 1 for both trials separately. Statistically significant equivalence between the 2 sampling methods was proven in both trials (with p < 0.0001 within an equivalence range of ± 0.5 log cfu/ml, respectively). The field trial revealed TVC on carcass surfaces sometimes at or below the lower detection limit for the ACM, while TVC from all carcasses were able to be determined by WDSM. Overall, low contamination levels were less reliably detectable by ACM than by WDSM. The ACM can be seen as an additional and suitable sampling procedure for pig skin and can contribute to the identification of abattoir specific risk factors for investigations of the hygienic status at process stages along the pig slaughter line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microbiological criteria for foodstuffs, as defined in Regulation No 2073/2005 (EC 2005) for process hygiene, are suitable to verify the hygienic status of food at the end of the slaughter line and to evaluate the process performance (EFSA 2007; Zweifel et al. 2014). Strict compliance with slaughter hygiene principles is of utmost importance as a measure to prevent surface contamination of the carcasses (Zweifel and Stephan 2003). In turn, the hygienic condition of the carcass surface is an essential prerequisite for the hygienic quality of meat products at the end of the slaughter line (Gisske and Klemm 1963). However, monitoring along the slaughter process at different stages is particularly useful to be able to identify specific risk factors and processing steps for contamination within the slaughter line and to correct them. The determination of total viable count (TVC) of bacteria as a general hygienic indicator (Otten 2005) is useful for orientation regarding the microbiological contamination of fresh meat (Snijders et al. 1984; Charlebois et al. 1991).
Regulation (EC) No 2073/2005 (EC 2005) requires compliance with ISO 17604:2015-12 (2015), which predetermines sampling methods to be used for carcass surfaces. Among the prescribed sampling methods, only a few are suitable for practical hygiene monitoring (Schulze 2000). For carcass sampling, destructive and nondestructive sampling methods can be used as described in ISO 17604:2015-12 (2015). For destructive methods, a defined tissue sample from the carcass is excised and used to determine the bacterial count (Otten 2005). Due its invasive and destructive nature and the time and expertise required, excision sampling is often impractical for meat processing plants (Gallina et al. 2015), but is preferred for process hygiene control of pig carcasses before chilling (Bolton 2003). The microbiological limits for pig carcasses are based exclusively on excision, but alternative methods can be used if they have been certified and validated as correlating with tissue excision (EC 2005). Non-destructive methods involve different swabbing methods and are used to sample larger carcass areas. The tested surface remains intact, and the carcass retains its full commercial value after sampling. (Schulze 2000). Following Louwers and Klein (1994), swabbing methods are based on absorbing bacteria from the sampled matrix and are divided into single swabbing and wet-dry double swabbing method (WDSM). Sampling procedure of the WDSM is quick (Zweifel and Stephan 2003), and sampling areas with low incidences and uneven distributions of bacteria can be covered (Capita et al. 2004). However, this precise sampling method requires expertise (Louwers and Klein 1994). Compared to single dry swabbing, WDSM provided higher bacteria recovery rates (Kleiner 2000) and is recommended (EC 2005) and widely used in the EU meat industry (Pepperell et al. 2005). A third surface sampling technique is the contact method, i.e., direct contact of solid agar on the testing surface. According to ISO 18593:2018 (2018), methods with contact plates such as Replicate Organism Direct Agar Contact (RODAC) are used for semi-quantitative microbiological analysis of fitments, equipment, and utensils. Sampling is quick and easy, and no other work materials are needed on site (Capita et al. 2004). Additionally, RODAC plates are suitable for microbiological sampling of carcass surfaces (Globisch et al. 1996; Kleiner and Hilgert 2004a, b). To enable quantitative colony counting, Baumgart and Kussmann (1975) and Kusch (1977) adapted the semiquantitative approach by, after sampling, homogenizing the agar slice in buffered peptone water (BPW) for preparation of a decimal dilution series and plating. They called this procedure the agar contact method (ACM).
Relatively few comparative studies of contact methods conducted on carcass surfaces exist (Snijders et al. 1984; Cordray and Huffman 1985; Kleiner and Hilgert 2004a, b), and studies focused on specific bacteria (Nortje et al. 1982; Fliss et al. 1991). Therefore, our study should show whether this practical, fast and easy sampling procedure could be a suitable method for studies of the hygienic status of slaughter pigs at process stages of the slaughter line, to detect abattoir specific risk factors. To this aim, we compared WDSM and ACM on laboratory-contaminated pig skin pieces and on pig carcasses in a field trial at an abattoir.
2 Material and methods
2.1 Study design
The study was designed in 2 parts: an experimental trial on laboratory-contaminated pig skin pieces and a field trial on pig carcasses at an abattoir. Each skin piece/carcass was sampled using the 2 different sampling methods, WDSM and ACM.
2.2 Sampling methods
For WDSM, the sampling procedure was adapted from ISO 17604:2015–12 (2015). First, a 20 cm2 sterile plastic template (COPAN, Brescia, Italy) was placed on the pig skin. In the experimental trial, this template was fixed by inserting 2 sterile cannulas (B. Braun Melsungen AG, Melsungen, Germany), and in the field trial, it was held manually on carcass surfaces. A commercial wet cotton swab (3 M Health Care, Saint Paul, USA) was first squeezed out on the inner wall of its tube containing 10 ml BPW by turning. The damp swab was rubbed firmly across the delineated skin/carcass surface at a 45° angle in a meandering pattern with slight rotation and with 7 strokes both horizontally and vertically. Afterwards the swab was moved along the inner edge of the template to ensure the skin here was sampled, and the swab was returned into the tube. A second dry cotton swab (COPAN, Brescia, Italy) was used in the same way, then placed in the same tube with BPW as the associated wet swab. Both swabs pooled together constituted one sample.
For ACM, commercial agar contact plates containing plate count agar (PCA) with a slightly raised agar surface of 23 cm2 (VWR International GmbH, Darmstadt, Germany) were used. Each agar plate was pressed firmly for 5 s on the surface without lateral movement and was closed again directly after sampling. The sampling was always performed by the same researcher.
2.3 Experimental trial with laboratory-contaminated pig skin pieces
For the experimental trial, in total 160 samples comprising 128 experimental test samples that were contaminated in the laboratory, and 32 status quo samples that were not contaminated in the laboratory, were collected from 32 pig skin pieces over 6 sampling days. The scalded, de-haired, and singed pig skin pieces (from neck, brisket, flank, rump, or back) were purchased from a local wholesaler. On the same day the laboratory work was to be conducted, pig skin pieces were cut from the chilled carcasses and transported in a plastic bag to the laboratory under constant refrigeration at 4 °C. Each pig skin piece was cut into approximately 50 cm × 40 cm pieces and marked with 5 equally-sized fields for sampling. Two fields were marked for ACM by using a sterile stainless-steel round die cutter (diameter 50 mm) outlined with a food marker pen. Two fields for WDSM were delineated using a sterile plastic template (COPAN, Brescia, Italy). One field was used for status quo sampling by WDSM. From the 5 marked fields, 4 were contaminated as described below. The order of distribution of contaminated fields was changed randomly for all pig skin pieces.
Artificial contamination of the pig skin was performed using 2 concentrations of reference bacteria originating from DSMZ (German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany) and ATCC (American Type Culture Collection, Manassas, USA). The following different species, commonly found on pig carcass surfaces (Corbeil 2014), were used to create the bacteria suspension: Escherichia coli DSMZ 1103; Enterococcus faecalis DSMZ 2570; Staphylococcus aureus DSMZ 799; Micrococcus luteus DSMZ 20030; Streptococcus suis DSMZ 9682; Pseudomonas aeruginosa ATCC 15442. Each organism was prepared by suspending one colony forming unit (cfu) in 10 ml of Brain–Heart-Infusion broth (Oxoid Ltd, Hampshire, UK). After suspensions were incubated for 24 h at 30 °C (P. aeruginosa) or at 37 °C (other bacteria), volumes of 2.5 ml (E. coli) or 5 ml (other bacteria) were mixed to prepare the bacteria suspension for artificial contamination. The bacteria suspension was diluted so it contained up to 7 log cfu/ml using sodium chloride peptone broth (Merck KGgA, Darmstadt, Germany), confirmed by plating on PCA (Th. Geyer GmbH & Co. KG, Renningen, Germany). The high concentration, containing 7 log cfu/ml, represented high-level contamination and the low concentration, containing 3 log cfu/ml, represented low-level contamination.
For both sampling methods, 100 µl of bacteria suspension was pipetted into the marked fields on the pig skin pieces at room temperature; the suspensions were repeatedly spread out with a stainless-steel spatula until they were visibly dry. Afterwards, sampling of the respective fields using WDSM (low-level and high-level contamination; status quo field without artificial contamination) and ACM (low- and high-level contamination) was performed (see 2.2).
2.4 Field trial at the abattoir
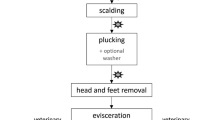
The field trial was conducted at an industrial pig abattoir located in Northwestern Germany with a slaughter capacity of 2900 pigs per day. The sampling point was at the end of the slaughter line post-evisceration and dressing but directly before the carcasses entered the chiller. In total, 58 samples from 29 slaughtered pigs from 9 different holdings were collected randomly. 3 carcasses from 8 holdings respectively and 5 carcasses from 1 holding were sampled. Each carcass was sampled in the perianal area, whereby one side was sampled using WDSM and the other side using ACM. The 2 sampling methods were used alternately on the carcass sides, starting from the first pig carcass being sampled by ACM on the left perianal side and by WDSM on the right perianal side. These sides were swapped for each subsequent pig carcass.
After sampling, pooled swabs and agar contact plates were transported at 4 °C to the institute´s laboratory. Microbiological examination started the next day.
2.5 Microbiological examination
WDSM tubes were vigorously shaken with a vortex mixer (Vortex-Genie 2 Vortex Mixer, Modell: G-560 E, Scientific Industries, INC., Bohemia, New York, USA) for 30 s before decimal solution series were prepared. For determination of TVC by ACM, each agar slice was dislodged from the Petri dish with sterile forceps and transferred into a sterile blender bag with lateral filter (VWR International GmbH, Darmstadt, Germany). Afterwards, a volume of 100 ml BPW (Merck KGgA, Darmstadt, Germany) was added, and the content of the blender bag was homogenized with a stomacher (Smasher™ High-Performance Blender/Homogenizer, bioMerieux, Marcy-l´Étoile, France) for 2 min with a speed of 560 strokes per minute. After homogenization, the resulting basic homogenate was used for the decimal dilution series. For quantitative analysis of TVC, duplicate 0.1 ml volumes from basic homogenate and each dilution series were dropped onto PCA and streaked out using a sterile loop (Sarstedt AG & Co. KG, Nümbrecht, Germany). Incubation under aerobic conditions at 30 °C was 48 ± 2 h for WDSM samples and 72 ± 2 h for ACM samples. After incubation, colonies were counted for each dilution step and the TVC were calculated. Colonies were also calculated in the case when only 1 colony was counted per plate in the minimum dilution.
2.6 Statistical analyses
The minimum required sample size of n = 26 was calculated with statistical software NCSS-PASS, Version 2021 (NCSS, LLC, Utah, USA), using a paired t-test for equivalence with a minimum power = 80%, α = 5%, confidence limits of ± 0.15 and σ = 0.25.
The weighted arithmetic mean TVC was calculated for each sample. Results as counted, in cfu/cm2, initially had skewed distributions, so all results were normalized by transforming via logarithms to the base 10. The minimum limit of detection (1 colony counted only) was 1.00 log cfu/cm2 for WDSM and 1.94 log cfu/cm2 for ACM. In the case of a complete lack of visible growth, for statistical test purposes, bacteria counts were considered as half of the minimum limit of detection, which was 0.70 log cfu/cm2 for WDSM and 1.64 log cfu/cm2 for ACM.
T-tests (two one-sided equivalence tests, TOST) were used to determine if means were significantly different. Following Hübner et al. (2002), a range of 0 ± 0.5 log cfu/cm2 for the mean difference was determined as a limit of equality. The level of significance was set at 0.025 for each one-sided test, yielding a total confidence limit of 95%.
Furthermore, linear regression models omitting any intercept were created and the coefficient of determination (R2) was used to assess how the data fitted the model. All statistical analyses were performed with SAS (SAS®, version 9.4, SAS Institute Inc., Cary, North Carolina, USA).
3 Results
3.1 Total viable counts and mean differences
In the experimental trial with artificially contaminated pig skin pieces, TVCs could be determined both for low- (3 log cfu/ml) and high- (7 log cfu/ml) level contamination. In the field trial, 9 samples taken using ACM showed no visible growth, and in 10 samples with the same method, only 1 colony could be counted.
In the experimental trial, for low-level contamination, the mean TVCs were 3.49 log cfu/cm2 for WDSM and 3.31 log cfu/cm2 for ACM. For high-level contamination, the mean TVC for WDSM was 6.06 log cfu/cm2 while it was 6.07 log cfu/cm2 for ACM. The mean difference between the 2 methods was − 0.18 log cfu/cm2 for low-level and 0.01 log cfu/cm2 for high-level contamination. For the field trial, the mean TVCs were 2.05 log cfu/cm2 for WDSM, and 2.04 log cfu/cm2 for ACM with a mean difference of − 0.01 log cfu/cm2 (Fig. 1; Table 1).
The mean TVC of the 32 pig skin pieces from the status quo fields that had not been artificially contaminated in the laboratory was 3.47 log cfu/cm2, which was in the range of the mean TVC of low-level contamination.
3.2 Equivalence tests and linear regression models
Statistical equivalence between WDSM and ACM was proven by the equivalence tests in all experiments, as the confidence intervals calculated for mean differences fell fully within the acceptable range of 0 ± 0.5 log cfu/cm2 (p < 0.0001 for both trials; Table 2). The linear regression models with log cfu/cm2 ACM as the dependent variable and log cfu/cm2 WDSM as the explaining variable showed the strong relation between the 2 methods, since values for R2 exceeded 90% and slope parameters were nearly 1 for all models (Fig. 2; Table 3).
4 Discussion
In our investigation, the incubation times of 48 ± 2 h for WDSM samples and 72 ± 2 h for ACM samples yielded statistically equivalent results for the 2 methods (Table 2). Therefore, these results can be considered as comparable, and all results can be compared with each other. Similarly, Salo et al. (2000), who investigated incubation times of 48 h and 72 h for WDSM and ACM, respectively, reported there were no statistically significant differences in TVCs.
For our experimental trial, the decision was made to contaminate the pig skin surfaces in the laboratory so that the number of bacteria was known and similar on all skin parts. From our point of view, this was necessary, because studies have shown that different parts of the carcass surface contain different levels of contamination (Ghafir and Daube 2008), and contamination is likely to be variable because some areas of the carcass surface are more susceptible to contamination than others (Beneke et al. 2011). To minimize this influencing factor and to avoid the influence of any natural but non-homogenous skin contamination, the pig skin pieces were contaminated (Pepperell et al. 2005) with a homogenous solution of bacteria species that are commonly found in pig skin microbiota (Corbeil 2014) in 2 pre-determined concentrations. The low-level and high-level contamination, with 3 log cfu/ml and 7 log cfu/ml, respectively, were chosen because various TVC values can be found on pig carcasses at the different processing stages along the pig slaughter line (Wheatley et al. 2014). Comparing the results of both contamination levels with the initial status quo contamination on the pig skin pieces, the initial microbiota counts were in range of the TVC with low-level contamination. This likely indicates the influencing factor of status quo contamination on the low-level TVC results.
TVCs measured by ACM and WDSM were statistically equivalent for all trials, shown by sampling contaminated pig skin pieces less than 1 h after inoculation. This is in line with results reported by Oberhäuser (2005), who sampled 5 min after application of artificial contamination. In general, bacterial transfer occurs directly from a contaminated surface to the agar surface of agar contact plates, and therefore, the recovery rate depends on the adhesion and detachability of bacteria (Schulze 2000; Capita et al. 2004). According to Notermans et al. (1991), the adsorption process of bacteria is reversible and they can still be removed by washing, since 2 to 3 h after application of an inoculum firm adhesion to the skin is not formed (Firstenberg-Eden et al. 1979). However, in our field trial, mean TVCs for 19 carcasses sampled by ACM were at or below the lower detection limit, with only 1 or no colonies detected. At the sampling position at the abattoir, only low numbers of bacteria on the carcasses could be expected (Moura-Alves et al. 2022). This, together with the results of our experimental trial with low-level contamination, shows the limitation of ACM for detecting low-level contamination on pig skin/carcass surfaces at the abattoir. Since WDSM was able to determine TVC values in all samples and the TVCs were equivalent between the 2 sampling methods, WDSM is more suitable than ACM for determining the TVC of freshly slaughtered pig carcasses.
In accordance with other studies (Baumgart and Kussmann 1975; Kusch 1977), we showed that representative TVCs in the case of our high-level contamination on pig skin were obtained with ACM in the experimental trial. Therefore, we conclude that ACM is useful for sampling in pig slaughter process where high bacterial contamination levels are expected, i.e., at the beginning of the slaughter line. However, our study showed ACM is not suitable for sampling at the end of the pig slaughter line, where low bacterial loads should occur (Wheatley et al. 2014). Snijders et al. (1984) concluded that ACM is not appropriate for assessing carcass contamination. However, they used agar plates with a contact surface of only 7.5 cm2 compared to our 23 cm2 sampling area. Additionally, a contact surface that is too small leads to greater variation of the results, depending on the surface being examined (Louwers and Klein 1994). The sampling area used in this study (23 cm2) for ACM was large enough to yield comparable results to WDSM and so is appropriate for pig carcass surface sampling when high bacterial loads are expected.
For the low-level contamination of pig skin pieces in our study, the mean TVC was 0.18 logs higher for WDSM than for ACM. Also results of our field trial, with a mean difference of just 0.1 logs between mean TVCs measured by the 2 methods, are in agreement with other comparative studies (Kleiner and Hilgert 2004a, b), where mean TVCs differed by up to 0.5 logs based on 20 cm2 for WDSM and RODAC methods. In contrast to our results, Cordray and Huffman (1985) found lower bacteria recovery using the semiquantitative RODAC method than when using dry swabbing on chilled pig carcasses. This discrepancy with our results for freshly slaughtered pigs can possibly be explained by the chill storage period, which typically leads to a decrease of microbial loads (Capita et al. 2004). On the moist sampling surfaces of freshly slaughtered pig carcasses, WDSM recovered higher TVCs than were determined on dry carcasses (Anderson et al. 1987). Since we used both WDSM and ACM with quantitative determination of TVCs, our results are more comparable between our 2 methods than results from swabbing techniques compared to the semi-quantitative results of the RODAC method, i.e., by Cordray and Huffman (1985). The only limitation is that WDSM is more suitable for lower carcass contamination levels than ACM.
Finally, our comparative study showed high concordance between the results of ACM and WDSM for determining TVC on pig skin surfaces. For each part of the examination, statistical equivalence of the results was proven by t-tests, and the linear regression models showed an excellent fit between the compared methods. The differences between TVCs generated by WDSM and ACM were ≤ 0.5 log, which can be expected for quantitative microbiological measurement methods in a laboratory and are considered as indicating equivalence (Hübner et al. 2002).
5 Conclusion
ACM and WDSM produced comparable TVCs, but ACM had a higher minimum limit of detection than WDSM. This relatively higher limit of detection must be taken into account if ACM is chosen as an appropriate technique for pig carcass sampling to determine the hygienic status (indicated by TVC) of contaminated pig carcasses. Therefore, we suggest that ACM can be used for individual cases and only for processing stages where relatively high carcass contamination and high resulting bacterial loads on the carcass are expected. Thus, ACM is suitable for sampling live pigs in the lairage area, and for sampling on the slaughter line without work interruptions, as it is a very quick sampling technique.
References
Anderson ME, Huff HE, Naumann HD, Marshall RT, Damare J, Johnston R, Pratt M (1987) Evaluation of swab and tissue excision methods for recovering microorganisms from washed and sanitized beef carcasses. J Food Prot 50:741–743. https://doi.org/10.4315/0362-028X-50.9.741
Baumgart J, Kussmann H (1975) A spray method for determining the surface bacterial content of animals for slaughter. Fleischwirtschaft 55:1113–1114
Beneke B, Klees S, Stuhrenberg B, Fetsch A, Kraushaar B, Tenhagen BA (2011) Prevalence of methicillin-resistant Staphylococcus aureus in a fresh meat pork production chain. J Food Prot 74:126–129. https://doi.org/10.4315/0362-028X.JFP-10-250
Bolton DJ (2003) The EC decision of the 8th June 2001 (EC/471/2001): excision versus swabbing. Food Control 14:207–209. https://doi.org/10.1016/s0956-7135(02)00093-2
Capita R, Prieto M, Alonso-Calleja C (2004) Sampling methods for microbiological analysis of red meat and poultry carcasses. J Food Prot 67:1303–1308. https://doi.org/10.4315/0362-028x-67.6.1303
Charlebois R, Trudel R, Messier S (1991) Surface contamination of beef carcasses by fecal coliforms. J Food Prot 54:950–956. https://doi.org/10.4315/0362-028X-54.12.950
Corbeil J (2014) Microbiological status of porcine slaughter by-products in consideration of market regulations. Dissertation, Ludwig-Maximilians-Universität München. https://doi.org/10.5282/edoc.16760
Cordray JC, Huffman DL (1985) Comparison of three methods for estimating surface bacteria on pork carcasses. J Food Prot 48:582–584. https://doi.org/10.4315/0362-028x-48.7.582
EC (2005) European Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. https://eur-lex.europa.eu/eli/reg/2005/2073/oj (current consolidated version: 08/03/2020). Accessed 03 July 2023
EFSA (2007) Opinion of the Scientific Panel on biological hazards (BIOHAZ) on microbiological criteria and targets based on risk analysis. EFSA J 5:462. https://doi.org/10.2903/j.efsa.2007.462
Firstenberg-Eden R, Notermans S, Thiel F, Henstra S, Kampelmacher EH (1979) Electron microscopic investigations into attachment of bacteria to teats of cows. J Food Prot 42:305–309. https://doi.org/10.4315/0362-028x-42.4.305
Fliss I, Simard R, Ettriki A (1991) Comparison of three sampling techniques for microbiological analysis of meat surfaces. J Food Sci 56:249–250
Gallina S, Bianchi DM, Ru G, Maurella C, Barzanti P, Baioni E, Virgilio S, Mioni R, Lanni L, Migliazzo A, Losio MN, Bove D, Scuota S, Goffredo E, Decastelli L (2015) Microbiological recovery from bovine, swine, equine, and ovine carcasses: comparison of excision, sponge and swab sampling methods. Food Control 50:919–924. https://doi.org/10.1016/j.foodcont.2014.10.052
Ghafir Y, Daube G (2008) Comparison of swabbing and destructive methods for microbiological pig carcass sampling. Lett Appl Microbiol 47:322–326. https://doi.org/10.1111/j.1472-765X.2008.02433.x
Gisske W, Klemm G (1963) Bacterial content of the skin of meat hogs as affected by various scalding methods. Fleischwirtschaft 15:288–292
Globisch H, Wilkens S, Jacob A, Thien J (1996) Anwendbarkeit von Abklatschverfahren für die Untersuchung von Oberflächenkeimgehalten bei Schlachttierkörpern: Vergleichende Bestimmung der aeroben mesophilen Gesamtkeimzahl mittels Abklatschtechnik und destruktiver Probenahmetechnik. Fleischwirtschaft 76:1116–1118
Hübner P, Gautsch S, Jemmi T (2002) In house Validierung (single laboratory validation) of microbiological methods. Mitt Lebensm Hyg 93:118–139
ISO 17604:2015–12 (2015) Microbiology of the food chain-Carcass sampling for microbiological analysis. International Organization for Standardization, Geneva. https://doi.org/10.31030/2305385
ISO 18593:2018 (2018) Microbiology of the food chain–Horizontal methods for surface sampling. International Organization for Standardization, Geneva. https://doi.org/10.31030/2833890
Kleiner U (2000) The power of statement of swabs with the scope of hygiene controls. Fleischwirtschaft 80:118–121
Kleiner U, Hilgert S (2004a) Conversion of the decision 2001/471/EC: comparison of the destructive and non-destructive sampling techniques for microbiological control of meat surfaces – 1. Swine carcase. Fleischwirtschaft 84:101–104
Kleiner U, Hilgert S (2004b) Conversion of the decision 2001/471/EC: Comparison of the destructive and non-destructive sampling techniques for microbiological control of meat surfaces – 2. Sections of swine. Fleischwirtschaft 84:146–149
Kusch D (1977) Ein Beitrag zur Hygienekontrolle in fleischverarbeitenden Betrieben. J Food Saf Food Qual 28:68–71
Louwers J, Klein G (1994) Suitability of sampling methods for the investigation of the environment in EC-licensed meat rendering and processing plants. Berl Muench Tieraerztl Wochenschr 107:367–373
Moura-Alves M, Carvalho M, Baggio Ribeiro DH, Barbosa J, Silveira L, Pista A, Pinto HP, Saraiva C, Teixeira P, Esteves A (2022) Hygiene indicators and salmonellae on surfaces of swine carcasses from two slaughterhouses in northern Portugal. J Food Prot 85:1566–1575. https://doi.org/10.4315/JFP-21-312
Nortje GL, Swanepoel E, Naude RT, Holzapfel WH, Steyn PL (1982) Evaluation of three carcass surface microbial sampling techniques. J Food Prot 45:1016–1017. https://doi.org/10.4315/0362-028X-45.11.1016
Notermans S, Dormans J, Mead G (1991) Contribution of surface attachment to the establishment of micro-organisms in food processing plants: a review. Biofouling 5:21–36. https://doi.org/10.1080/08927019109378226
Oberhäuser K (2005) Comparison of pour upon method and wet-dabbing method to judge the disinfecting qualities of building materials. Dissertation, Freie Universität Berlin. https://doi.org/10.17169/refubium-13050
Otten K (2005) Praktische Umsetzung der Entscheidung 2001/471/EG zur Hygienekontrolle in einem mittelständischen Direktvermarkterbetrieb. Dissertation, Justus-Liebig-Universität Gießen. http://geb.uni-giessen.de/geb/volltexte/2006/2694/. Accessed 03 July 2023
Pepperell R, Reid CA, Solano SN, Hutchison ML, Walters LD, Johnston AM, Buncic S (2005) Experimental comparison of excision and swabbing microbiological sampling methods for carcasses. J Food Prot 68:2163–2168. https://doi.org/10.4315/0362-028x-68.10.2163
Salo S, Laine A, Alanko T, Sjöberg AM, Writanen G, Guðbjörnsdóttir B, Jessen B, Langsrud S, Lindquist K, Lundén J, Mäki M, Nerbrink E, Niclasen Ó, Tuominen P, Tuompo H, Vatunen E, Woivalin A (2000) Validation of the microbiological methods Hygicult dipslide, contact plate, and swabbing in surface hygiene control: a nordic collaborative study. J AOAC Int 83:1357–1366. https://doi.org/10.1093/jaoac/83.6.1357
Schulze G (2000) The representativeness of RODAC technique. Dissertation, Freie Universität Berlin
Snijders JMA, Janssen MHW, Gerats GE, Corstiaensen GP (1984) A comparative-study of sampling techniques for monitoring carcass contamination. Int J Food Microbiol 1:229–236. https://doi.org/10.1016/0168-1605(84)90019-9
Wheatley P, Giotis ES, McKevitt AI (2014) Effects of slaughtering operations on carcass contamination in an Irish pork production plant. Ir Vet J 67:1–6. https://doi.org/10.1186/2046-0481-67-1
Zweifel C, Stephan R (2003) Microbiological monitoring of sheep carcass contamination in three Swiss abattoirs. J Food Prot 66:946–952. https://doi.org/10.4315/0362-028x-66.6.946
Zweifel C, Capek M, Stephan R (2014) Microbiological contamination of cattle carcasses at different stages of slaughter in two abattoirs. Meat Sci 98:198–202. https://doi.org/10.1016/j.meatsci.2014.05.029
Acknowledgements
The authors thank the staff of the abattoir for the support and assistance with sampling.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. This study was conducted within the joint research project KontRed, which was supported by funds of the Federal Ministry of Food and Agriculture based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food under the innovation support program (Grant number: 281C104A18).
Author information
Authors and Affiliations
Contributions
DM and NL conceived the first study conception. All other authors contributed to the final study conception and design. Material preparation, data collection and laboratory analysis were performed by RF. Statistical analyses were performed by RF, LK and JGK. The first draft of the manuscript was written by RF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fürstenberg, R., Meemken, D., Langforth, S. et al. Comparison of the agar contact method and the wet-dry double swabbing method for determining the total viable bacterial count on pig carcass surfaces. J Consum Prot Food Saf 19, 41–48 (2024). https://doi.org/10.1007/s00003-023-01473-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-023-01473-6