Abstract
The concentrations of dioxins [polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs)], and dioxin-like polychlorinated biphenyls (DL-PCBs) in Atlantic herring depend on the fishing area. These substances originate from various anthropogenic sources and accumulate in the environment and in food. The influence of country-specific contaminant concentrations on human dietary exposure was studied exemplary for herring to show the influence of fish origin. PCDD/F and DL-PCB concentrations in herring from the Norwegian Sea and the Baltic Sea were combined with country-specific herring consumption. Herring concentrations showed geographical variation. For herring consumers, the 50th percentile dietary exposure to the total sum of PCDD/Fs and DL-PCBs amounted to 1.2 and 8.9 pg WHO-2005-TEQ/kg BW/week for Norway and Germany, respectively. The different exposure was mainly related to higher concentrations in herring from the Baltic Sea, rather than in herring from the Norwegian Sea. If contaminant concentrations are influenced by geographical origin, this should be integrated into the dietary exposure assessments. For herring, relevant fishing areas should be integrated into the sampling strategy to generate concentration data. The usage of country-specific data could refine exposure assessments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Contaminant concentrations can be related to geographical origin, or in the case of fish to fishing areas (Fechner et al. 2019a; b; c). For fish, variation in contaminant concentrations between different geographical areas has been shown (Azad et al. 2019; Frantzen et al. 2011; Karl et al. 2002; Karl and Lahrssen-Wiederholt 2013; Sunderland et al. 2018). Atlantic herring (Clupea harengus), in the following called herring, was selected for this case study because it is consumed in Norway and Germany, and because country-specific data on dioxins, i.e. polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), and dioxin-like polychlorinated biphenyls (DL-PCBs) are available. Both the Norwegian Sea and Baltic Sea are typical fishing areas (ICES 2017). Highest PCDD/F and DL-PCB concentrations in herring have been reported for the Baltic Sea (Airaksinen et al. 2014; Fernandes et al. 2018; Frantzen et al. 2011; Karl and Lahrssen-Wiederholt 2013; Strucinski et al. 2013).
Discharge of PCDD/Fs and DL-PCBs originates from various anthropogenic sources. These contaminants have entered the food chain and are still ubiquitous present despite their banned use (Stockholm Convention and UNEP 2013). Fatty fish can be one of the main contributors to PCDD/F and DL-PCB exposure from food (Frantzen et al. 2011; Nøstbakken et al. 2021; Schwarz et al. 2014).
Substances investigated in this study are the 17 PCDD/F congeners that are persistent in humans, and the 12 DL-PCBs with similar toxicological properties (EC 2011; EPA 2008). The toxic equivalent (TEQ) concept updated in 2005 is used to estimate the combined toxicity of PCDD/Fs and DL-PCBs mixtures through the toxic equivalency factors (TEFs) (EFSA et al. 2018; EPA 2008; Van den Berg et al. 2006). In order to protect consumers, the EU maximum levels in muscle meat of fish are 3.5 pg WHO-2005-TEQ/g for the sum of 17 PCDD/Fs, and 6.5 pg WHO-2005-TEQ/g for the total sum of 29 PCDD/Fs and DL-PCBs (EC 2011).
According to the European Food Safety Authority (EFSA), 2 pg WHO-2005-TEQ per kg body weight (BW) per week is a tolerable weekly intake (TWI) (EFSA et al. 2018). To assess the dietary exposure, pooled European concentrations and country-specific consumption data were used by EFSA. This study aimed to perform an exemplary chronic dietary exposure assessment of PCDD/Fs and DL-PCBs from herring, using country-specific concentrations combined with country-specific consumption.
2 Materials and method
2.1 Country-specific dietary exposure assessment
For dietary exposure assessments, consumption data based on surveys and monitoring data on contaminant concentrations in food are typically used (EFSA et al. 2018; Fechner et al. 2019b, c; Sarvan et al. 2017; VKM 2014; WHO and FAO 2009). To perform a chronic dietary exposure assessment on PCDD/Fs and DL-PCBs in herring from Norway and Germany, we used country-specific consumption and concentration data. Data were derived from a project that studied (1) herring consumption and (2) substance concentrations in herring for exposure assessment (Fechner et al. 2019a). For Norway and Germany, the consumption in g/day related to BW in kg derived as individual mean of two 24-h recalls was combined with lower bound (LB) and upper bound (UB) mean concentrations in pg/g (Fig. 1). The LB and the UB approach is used to include samples with concentrations below the limit of detection (LOD) or limit of quantification (LOQ) in the exposure modelling using the limits given for the respective sample. For LB concentrations, results below the LOD or the LOQ were replaced by zero. For UB concentrations, results below the LOD were replaced by the LOD, results below the LOQ were replaced by the LOQ. Concentrations and exposure in the current sturdy were always expressed for the whole weight of herring fillet based on WHO-2005-TEQs. The herring consumption was estimated for all participants in the consumption surveys (both consumers and non-consumers) and for the sub-group of herring consumers only. The dietary exposure to PCDD/Fs and DL-PCBs was compared to the TWI of 2 pg TEQ/kg BW/week (EFSA et al. 2018).
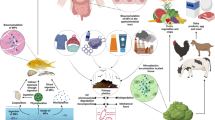
Calculation of dietary exposure (WHO and FAO 2009) used for the determination of the chronic dietary intake of PCDD/Fs and DL-PCBs from herring in Norway and Germany. PCDDs polychlorinated dibenzo-p-dioxins, PCDFs polychlorinated dibenzofurans, DL-PCBs dioxin-like polychlorinated biphenyls, WHO World Health Organization, FAO Food and Agricultural Organization of the United Nations
Data on herring consumption were derived from the Norwegian Norkost 3 (n = 1787) conducted during 2010–2011 (Totland et al. 2012) and the German National Nutrition survey II (NVS II) (n = 13,926) conducted during 2005–2006 (Brombach et al. 2006; Krems et al. 2006). Participants’ age ranged from 18 to 70 years in Norway and from 14 to 80 years in Germany. Individual means of two 24-h recalls were derived. Norwegian consumption data were available as aggregated foods (i.e. amount of herring fillet in combination with other ingredients like onions in pickled herring) and in a disaggregated version as amount of herring fillet. For Germany, disaggregated consumption amounts were available, i.e. household recipes including the amount of herring fillet, but industrial herring products were not disaggregated and the amount of consumed herring included other ingredients like onion (Fechner et al. 2019a).
2.2 Statistics and calculations
For statistical analyses, IBM® SPSS® Statistics 25 was used. There, the PTILE command was used within the CTABLES command in order to determine P95 as a parameter for concentrations, consumption and dietary exposure. Microsoft® Excel 2016 was used to prepare figures.
3 Results
Dietary exposure to PCDD/Fs and DL-PCBs from herring consumption was investigated in both the sub-group of herring consumers and in all participants (consumers and non-consumers) based on consumption surveys in Norway and Germany (Fig. 2). The concentrations and exposure from herring of different geographical origin showed clear differences that are not only related to consumption behaviour. Herring consumption was similar for all German participants (14–80 years) and the subset of German participants between 18 and 70 years were appropriate to the Norwegian survey (Online Resource (OR) 1–2). Therefore, the evaluations of this study cover all age ranges of the surveys.
Human chronic dietary exposure of 17 PCDD/Fs (dioxins), 12 DL-PCBs and the total of PCDD/Fs and DL-PCBs for herring consumers based on the consumption surveys (upper graphs) and for all participants in the consumption surveys (lower graphs). PCDDs polychlorinated dibenzo-p-dioxins, PCDFs polychlorinated dibenzofurans, DL-PCBs dioxin-like polychlorinated biphenyls, WHO World Health Organization, TEQ toxic equivalents, BW body weight, LB lower bound, UB upper bound, P50 50th percentile, P95 95th percentile, TWI tolerable weekly intake
In Norway, 99 participants (5.5%) consumed herring, while in Germany 596 participants (4.3%) reported herring consumption. Herring consumers represented a small percentage of the population. For herring consumers, the P50 UB dietary exposure to the total sum of PCDD/Fs and DL-PCBs from herring was 1.2 and 8.9 pg TEQ/kg BW/week for Norway and Germany, respectively. This was based on UB concentrations and disaggregated consumption. Assuming that only herring from the sampled fishing areas are consumed, which is the Baltic Sea in Germany and the Norwegian Sea in Norway, German P50 consumers and persons with a higher consumption and Norwegian high consumers (P95) exceeded the TWI of 2.0 pg TEQ/kg BW/week for PCDD/Fs and DL-PCBs (EFSA et al. 2018).
When all participants (both herring consumers and non-consumers) were included, the exposure to the total sum of PCDD/Fs and DL-PCBs ranged between 0 pg TEQ/kg BW/week in P50 estimates and 1.0 pg TEQ/kg BW/week in P95 estimates for Norway and Germany, respectively. In both countries, the exposure for all participants was well below the TWI of 2.0 pg TEQ/kg BW/week (EFSA et al. 2018) (Fig. 2). Mostly, the P50 and the P95 exposure was estimated to 0 pg TEQ/kg BW/week, as most of the participants in the surveys were non-consumers of herring. For Norway, the P95 exposures to the total sum of PCDD/Fs and DL-PCBs were between 0.5 and 1.0 pg TEQ/kg BW/week (P95 LB for disaggregated consumption and P95 UB for aggregated consumption). This relates to herring consumption, as a P95 consumption could be derived because 5.5% of all participants from Norway were consumers while for Germany, the P95 consumption was 0 because less than 5% of the participants consumed herring.
Exposure estimates by gender and age are shown in the Supplementary Material (OR 1–2). For all participants (herring consumers and non-consumers), both countries showed the highest exposure of PCDD/Fs and DL-PCBs for the elderly (65–70 years) or the very elderly (from 75 years). Among herring consumers, there was a more even distribution among the different groups. In OR 3, the country-specific concentrations used in this study were compared to the pooled European concentrations used by EFSA as this database could substantially influence the exposure assessment.
4 Discussion
Chronic dietary exposure to PCDD/Fs and DL-PCBs from herring showed clear differences for consumers in Norway and Germany, as the results are based on data from herring samples from the Baltic Sea in Germany and from the Norwegian Sea in Norway. For Norwegian consumers of herring, the P50 UB dietary exposure to the total sum of PCDD/Fs and DL-PCBs from herring was 1.2 pg TEQ/kg BW/week (Fig. 2). However, the highest P50 UB estimate for the German herring consumers was 8.9 pg TEQ/kg BW/week (Fig. 2). German herring consumers had a higher exposure for 2 reasons: (1) higher mean concentrations of total PCDD/Fs and DL-PCBs in herring from the Baltic Sea (1.8 pg TEQ/g) vs. the Norwegian Sea (0.8–0.9 pg TEQ/g) (Fig. 1; Supplementary Material OR 3), and (2) higher mean herring consumption for German consumers (disaggregated: 0.88 g/day/kg BW) vs. Norwegian consumers (disaggregated: 0.31 g/day/kg BW) applied (Fechner et al. 2019a).
For all participants, the exposure from herring was very low, as only a low percentage of participants reported herring consumption (5.5% in Norway and 4.3% in Germany). The percentage of consumers could be underestimated, as only two 24-h recalls were available for the evaluation, meaning that possible herring consumptions on other days were not captured by this method.
EFSA calculated the total dietary exposure to PCDD/Fs and DL-PCBs for all participants of European surveys (consumers and non-consumers) using pooled European concentrations in food (EFSA et al. 2018). For the total sum of PCDD/Fs and DL-PCBs, the LB mean exposure ranged between 2.10 and 14.84 pg TEQ/kg BW/week and the UB P95 exposure ranged between 6.51 and 46.41 pg TEQ/kg BW/week depending on different age groups (EFSA et al. 2018). Considering all participants of consumption surveys, the results of EFSA are in line with the findings of the current study, as the estimates based on herring as single food of the current study are lower than the total dietary exposure based on various foods estimated by EFSA. EFSA did not provide exposure estimates for single food items or for herring consumers only, and a direct comparison with the results of the current study for herring consumers is not possible. Table 1 provides an overview of exposure estimates related to herring of the current study and total dietary exposure of the EFSA.
A benefit-risk assessment of fish from Norway used the same consumption data as in the current study (Norkost 3) and concentration data from 2006 to 2007 (VKM 2014). The exposure to the total sum of PCDD/Fs and DL-PCBs from herring was estimated for all participants and ranged between 0.07 and 0.42 pg WHO-1998-TEQ/kg BW/week (VKM 2014). This is similar to the findings of this study (Fig. 2). A benefit-risk assessment of Baltic herring highlighted that the consumption of smaller herring would reduce the exposure to organic contaminants (Tuomisto et al. 2020), thus geographical origin of fish might be only one factor in an overall assessment of benefits and risks. The preparation of herring could also substantially influence the contaminant concentration and this could be considered in the future by using concentration data of total diet studies (TDSs), in TDSs foods are prepared according to typical consumption habits in the respective country.
A study on dietary exposure to PCDD/Fs and DL-PCBs in Germany used consumption data of the same survey and concentration data from 2000 to 2010 (Schwarz et al. 2014). The exposure from food for average consumers and high-end consumers was 14.77 and 24.92 pg WHO-1998-TEQ/kg BW/week for the total sum of PCDD/Fs and DL-PCBs. Fish was identified as one of the most contributing food groups to total dietary exposure (Schwarz et al. 2014). The estimates of this study are similar to the dietary exposure for herring alone estimated for German herring consumers in this study (Fig. 2).
In the current study, the calculated exposure to PCDD/Fs and DL-PCBs from herring intake could be overestimated or underestimated due to the two 24-h recalls used for extrapolation to long-term consumption, which represents a typical uncertainty in exposure assessments (Fechner et al. 2019a). On the one hand, those who reported eating herring on both recall days would most likely not eat herring on a daily basis (over-reporting). On the other hand, herring is eaten once in a while, and the mean herring consumption in all participants can be underestimated since the number of consumers could be higher than the two captured recalls. As for Norwegian and German exposure calculations, the consumption data of two 24-h recalls were used, the uncertainty amounts the same.
For exposure scenarios of the current study, high concentrations (P95) were not used, as (1) the underlying assumption was to consume fish from only one fishing area because of the country-specific concentration data available and (2) consumption of only high contaminated herring from one fishing area was considered too specific and not realistic. Knowledge on the share of available fishing areas of herring on the market and area-related concentrations would be important for dietary exposure assessment, as taking the mean concentration of all fishing areas or of just a single fishing area could under- or overestimate the intake (Karl and Lahrssen-Wiederholt 2013).
To reduce uncertainties, the current exposure model needs to have refined consumption and concentration data as described by Fechner et al. (2019a). For the current study, concentration data were only available from single years and sampling was not representative for fishing areas. For exposure assessments, complex data are needed but available data are often limited, as sampling has its restrictions. Missing information on fishing season, fat content, age, size and other relevant fishing areas of herring could not be integrated in the model.
The use of country-specific data in the current study led to clear differences in dietary exposure for Norway and Germany, especially if consumers of herring were evaluated. Country-specific consumption is already considered in European exposure assessments (EFSA et al. 2018). Including country-specific concentrations and fishing areas of herring into the exposure assessment helped to evaluate the country-specific PCDD/F and DL-PCB intake. The exposure estimate could be refined by having more information on fishing areas. Our model was limited to the fishing areas in the Norwegian Sea and the Baltic Sea, which gives only a first insight into country-specific conditions, as other fishing areas are relevant as well (FAO 2022). This herring study exemplifies that integrating information on the geographical origin could help to differentiate dietary exposure estimates. This could be relevant for the consumption of other fish species and food in general in case of geographical variable contaminant concentrations.
5 Conclusion
The PCDD/F and DL-PCB exposure based on herring of different geographical origin showed clear differences that are not only related to consumption. For the small sub-group of herring consumers in the surveys, the P50 dietary exposure to the total sum of PCDD/Fs and DL-PCBs from herring was 1.2 and 8.9 pg TEQ/kg BW/week for Norway and Germany. When all participants (both herring consumers and non-consumers) were included in the surveys, the estimated dietary exposure was very low for Norway and Germany. More data on dioxin and DL-PCB concentrations in marketed herring based on fishing areas are needed for future evaluations.
The current study used concentrations of dioxins and DL-PCBs in herring from fishing areas available from standard national monitoring, with limitations in sampling. The same data are typically used for standard exposure assessments without considering the fishing area. This represents an uncertainty relevant for all assessments based on standard models or origin-related models.
Relations between the geographical origin of food and its contaminant concentrations could also be relevant for fish species other than herring or other foods. In comparison to higher pooled European concentrations, country-specific concentrations could refine exposure assessments. However, further knowledge on the geographical food origin and fishing areas available on the market is needed. In general, if substance concentrations are expected to show geographical variability, we recommend to integrate available geographical origins of marketed foods into the sampling strategy of monitoring programmes. Furthermore, food labelling would need to be extended to provide geographical origin information on more food products.
Data availability
We used data on food consumption and contaminant concentrations made available by the data owners free of charge. Original data could be requested from the data owner as referenced in the text.
References
Airaksinen R, Hallikainen A, Rantakokko P et al (2014) Time trends and congener profiles of PCDD/Fs, PCBs, and PBDEs in Baltic herring off the coast of Finland during 1978–2009. Chemosphere. https://doi.org/10.1016/j.chemosphere.2014.03.097
Azad AM, Frantzen S, Bank MS et al (2019) Effects of geography and species variation on selenium and mercury molar ratios in Northeast Atlantic marine fish communities. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.10.405
Brombach C, Wagner U, Eisinger-Watzl M, Heyer A (2006) Die Nationale Verzehrsstudie II. Ernährungs-Umschau 53(1): 4–9. https://www.ernaehrungs-umschau.de/fileadmin/Ernaehrungs-Umschau/pdfs/pdf_2006/01_06/EU01_04_09.pdf
EC, European Commission (2011) Commission Regulation (EU) No 1259/2011 of 2 December 2011. Official Journal of the European Union, 320: 18–23. https://eur-lex.europa.eu/eli/reg/2011/1259/oj
EFSA, CONTAM Panel (European Food Safety Authority Panel on Contaminants in the Food Chain), Knutsen HK, Alexander J et al (2018) Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. https://doi.org/10.2903/j.efsa.2018.5333
EPA, U. S. Environmental Protection Agency, Agency USEP (2008) Framework for application of the toxicity equivalence methodology for polychlorinated dioxins, furans and biphenyls in ecological risk assessment. Retrieved from https://www.epa.gov/sites/production/files/2013-09/documents/tefs-draft-052808-0804.pdf. Acessed 19 Aug 2022
FAO, Food and Agriculture Organization of the United Nations (2022) Fisheries and Aquaculture Department—species fact sheets—Clupea harengus. http://www.fao.org/fishery/species/2886/en. Acessed 19 Aug 2022
Fechner C, Frantzen S, Lindtner O, Mathisen GH, Lillegard ITL (2019a) Influence of the geographical origin on substance concentrations in herring as basis for dietary exposure assessments. EFSA J. https://doi.org/10.2903/j.efsa.2019.e170904
Fechner C, Greiner M, Heseker H, Lindtner O (2019b) Dietary exposure assessment of aluminium and cadmium from cocoa in relation to cocoa origin. PLoS One. https://doi.org/10.1371/journal.pone.0217990
Fechner C, Greiner M, Heseker H, Lindtner O (2019c) Refinement of dietary exposure assessment using origin-related scenarios. J Eposure Sci Environ Epidemiol. https://doi.org/10.1038/s41370-019-0117-6
Fernandes AR, Mortimer D, Holmes M et al (2018) Occurrence and spatial distribution of chemical contaminants in edible fish species collected from UK and proximate marine waters. Environ Int. https://doi.org/10.1016/j.envint.2018.02.047
Frantzen S, Måge A, Iversen SA, Julshamn K (2011) Seasonal variation in the levels of organohalogen compounds in herring (Clupea harengus) from the Norwegian Sea. Chemosphere. https://doi.org/10.1016/j.chemosphere.2011.06.034
ICES, International Council for the Exploration of the Sea (2017) Workshop on Stock Identification and Allocation of Catches of Herring to Stocks (WKSIDAC). https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2017/WKSIDAC/01_WKSIDAC%20Report%202017.pdf. Acessed 19 Aug 2022
Karl H, Lahrssen-Wiederholt M (2013) Factors influencing the intake of dioxins and dioxin-like PCBs via fish consumption in Germany. J Consum Prot Food Saf. https://doi.org/10.1007/s00003-013-0805-4
Karl H, Ruoff U, Bluthgen A (2002) Levels of dioxins in fish and fishery products on the German market. Chemosphere. https://doi.org/10.1016/s0045-6535(02)00399-5
Krems C, Bauch A, Götz A et al. (2006) Methoden der Nationalen Verzehrsstudie II. Ernährungs Umschau, 53(2): 44–50. https://www.ernaehrungs-umschau.de/fileadmin/Ernaehrungs-Umschau/pdfs/pdf_2006/02_06/EU02_44_50.pdf
Nøstbakken OJ, Rasinger JD, Hannisdal R et al (2021) Levels of omega 3 fatty acids, vitamin D, dioxins and dioxin-like PCBs in oily fish; a new perspective on the reporting of nutrient and contaminant data for risk–benefit assessments of oily seafood. Environ Int. https://doi.org/10.1016/j.envint.2020.106322
Sarvan I, Bürgelt M, Lindtner O, Greiner M (2017) Expositionsschätzung von Stoffen in Lebensmitteln: Die BfR-MEAL-Studie-die erste Total-Diet-Studie in Deutschland. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. https://doi.org/10.1007/s00103-017-2566-1
Schwarz MA, Lindtner O, Blume K, Heinemeyer G, Schneider K (2014) Dioxin and dl-PCB exposure from food: the German LExUKon project. Food Addit Contam Part A. https://doi.org/10.1080/19440049.2013.878041
Stockholm Convention and UNEP, United Nations Environment Programme (2013) Toolkit for Identification and Quantification of Releases of Dioxins, Furans and Other Unintentional POPs under Article 5 of the Stockholm Convention. Retrived from http://toolkit.pops.int/Publish/Main/Download.html. Acessed 19 Aug 2022
Strucinski P, Piskorska-Pliszczynska J, Maszewski S et al (2013) PCDD/Fs and DL-PCBs intake from fish caught in Polish fishing grounds in the Baltic Sea—characterizing the risk for consumers. Environ Int. https://doi.org/10.1016/j.envint.2013.03.002
Sunderland EM, Li M, Bullard K (2018) Decadal Changes in the Edible Supply of Seafood and Methylmercury exposure in the United States. Environ Health Perspect. https://doi.org/10.1289/EHP2644
Totland TH, Melnæs BK, Lundberg-Hallén N et al. (2012) Norkost 3. En landsomfattende kostholdsundersøkelse blant menn og kvinner i Norge i alderen 18–70 år, 2010–11. https://www.helsedirektoratet.no/rapporter/norkost-3-en-landsomfattende-kostholdsundersokelse-blant-menn-og-kvinner-i-norge-i-alderen-18-70-ar-2010-11/. Acessed 19 Aug 2022
Tuomisto JT, Asikainen A, Meriläinen P, Haapasaari P (2020) Health effects of nutrients and environmental pollutants in Baltic herring and salmon: a quantitative benefit-risk assessment. BMC Public Health. https://doi.org/10.1186/s12889-019-8094-1
Van den Berg M, Birnbaum LS, Denison M et al (2006) The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. https://doi.org/10.1093/toxsci/kfl055
VKM, Norwegian Scientific Committee for Food Safety (2014) Benefit-risk assessment of fish and fish products in the Norwegian diet—an update. https://vkm.no/download/18.2994e95b15cc54507161ea1a/1498222018046/0a646edc5e.pdf. Acessed 19 Aug 2022
WHO, World Health Organization and FAO, Food and Agriculture Organization of the United Nations (2009) Dietary exposure assessment of chemicals in food. In: IPCS, Inter-Organization Programme for the Sound Management of Chemicals (Ed.), Principles and Methods for the Risk Assessment of Chemicals in Food. http://apps.who.int/iris/bitstream/10665/44065/9/WHO_EHC_240_9_eng_Chapter6.pdf?ua=1. Acessed 19 Aug 2022
Acknowledgements
We thank the colleagues from the Norwegian Scientific Committee for Food and Environment (VKM) and the German Federal Institute for Risk Assessment (BfR) for their support during the project. The EU-FORA Fellowship Programme initiated by EFSA financed the project. Scientific evaluations do not necessarily represent the opinion of EFSA.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CF: project idea, concept, methodology, interpretation, statistical analysis, exposure assessment, manuscript draft. SF: methodology, access to data, interpretation, manuscript revision. OL: methodology, access to data, interpretation, manuscript revision. GHM: methodology, interpretation, manuscript revision. ITLL: project idea, concept, methodology, access to data, interpretation, manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fechner, C., Frantzen, S., Lindtner, O. et al. Human dietary exposure to dioxins and dioxin-like PCBs through the consumption of Atlantic herring from fishing areas in the Norwegian Sea and Baltic Sea. J Consum Prot Food Saf 18, 19–25 (2023). https://doi.org/10.1007/s00003-022-01401-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-022-01401-0