Abstract
The aim of this study was to describe the development and evaluation of a steam-mediated heating process for the sanitation of wooden boards used in cheese production. Wooden spruce fir boards from a cheese ripening centre were cut into small blocks and consecutively inoculated with suspensions of Listeria innocua at surface concentrations of 104 and 102 CFU/cm2, respectively. The inoculated blocks were stored at 12 °C and 94% relative humidity. Surface cell counts were determined after 24, 72, 144 and 240 h. Samples for the sanitising runs (steam treatment for 20 min with three different temperature programmes between 70 and 78 °C) were taken after 168, 192 and 216 h. Although a substantial decrease (>2 log CFU) in cell count occurred, L. innocua survived on the surface of the untreated wooden blocks for the entire timespan of the experiment (240 h), independent of the concentration of L. innocua in the inoculum. All three steam-mediated heating processes presented led to the Listeria-free hygienic status of the treated wood blocks. A comparison of abrasive (shavings) and swabbing (cotton rolls) sampling methods resulted in identical results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Wooden shelves have been used for many decades in traditional cheese production all over the world. Due to its porous structure, which makes it difficult to clean and disinfect, wood progressively shifted in the focus of a debate about its hygienic status and use in dairy operations (FDA 2014a). It is clear that utensils and other areas with contact to food surfaces must fulfil all legal requirements, such as EU Regulation 852/2004 (EC 2004a) on the hygiene of foodstuffs and EU Regulation 1935/2004 (EC 2004b) on materials and articles intended to come into contact with food, or the exigencies of the US Code of Federal Regulations 21CFR110.40a (FDA 2014b). To comply with all legal requirements, a food business operator accordingly has to prove that his cleaning and sanitising process for wooden shelves results in shelves that do not represent a source of contamination of pathogenic bacteria for the food coming into contact with it.
Listeria monocytogenes has been described as the most heat-resistant vegetative pathogen when present and grown in complex matrices that show elevated fat, protein or sodium chloride levels (Doyle et al. 2001) or in a low water activity (aw) environment (van Asselt and Zwietering 2006). In cheese production, heat resistance can be increased directly due to heat shock treatments, such as the heating of the curd, or indirectly, due to a general stress response after exposition to low aw environments such as brining processes (van Asselt and Zwietering 2006).
Concerning the risk of contamination with L. monocytogenes, one of the most important factors is the wooden shelving used in the process of cheese maturation. Above all, the advantage of using wooden ripening shelves lies in its moisture-retaining properties, which facilitates the control of the ripening process. However, regarding food safety, wooden boards may represent a hygienic risk. The porous structure of wood, in combination with elevated concentrations of fat, protein and sodium chloride brought into the food contacting area by the cheese coming from the brining process, may create an advantageous habitat for the eventual presence of pathogenic organisms such as L.monocytogenes if these are introduced as contaminants.
Many authors have reported the presence and persistence of L. monocytogenes in food processing environments (Abrihami et al. 1994; Carpentier and Cerf 2011; Carpentier and Chassaing 2004; Møretrø and Langsrud 2004). Between 1983 and 1987, Switzerland experienced a long-lasting outbreak of listeriosis, due to the contamination of a locally produced soft cheese. It caused at least 122 cases of human infection, of which 31 were fatal (Büla et al. 1995). One of the weak points in the cheese production process at that time was the individual cleaning process used on the wooden shelves in the concerned cheese production sites. In the aftermath of this outbreak, Agroscope’s Institute for Food Sciences (IFS) created a Listeria-specific consulting team and developed methods for sanitising wooden shelves via heat treatments equivalent to pasteurisation conditions. Recently, Zangerl et al. (2010) successfully proved the effectiveness of a sanitising process based on the submersion of wood blocks in hot water.
The aim of this study is to describe the development and evaluation of a steam-mediated heating process for the sanitising of wooden boards used in cheese production.
2 Materials and methods
2.1 Wooden boards
Solid wooden spruce fir boards (Picea abies, longitudinal cut, 100 × 30 cm, thickness 2 cm) used in the maturation of smear-ripened cheese were obtained from a cheese-ripening centre. The boards were delivered after a standard cleaning procedure (washing with detergents and mechanical brushing at 50 °C, then rinsing with cold water and storage in a special room for drying) used in the ripening centre. For the experiments, the shelves were cut into blocks measuring 7 × 7 cm. To eliminate unwanted bacteria, the blocks were pre-treated, unpressurised, in the autoclave at temperatures >90 °C for 20 min and allowed to cool down overnight to ambient temperatures before being inoculated with the test strain. The inoculation area of 25 cm2, a triangle arrangement of three closely spaced circles of 32 mm diameter including the central area in between the circles, was marked on the surface of the blocks with a pencil.
2.2 Test strain, inoculation of the blocks and sampling interval
For technical reasons, the sampling procedure occurred not in our laboratory, but in a specially prepared technical room. For safety reasons, therefore, Listeria innocua was determined as the test organism for the experiments of the evaluation process.
The evaluated strains of L. innocua originated from Agroscope’s internal strain collection. All tested strains were originally isolated from smeared cheese surfaces and chosen because of good growth and survival capacity as test organisms on cheese surfaces in other Agroscope projects. The D-values of four strains of L. innocua were determined using the method described by Peng et al. (2012); logDref- and z-values were calculated according to van Asselt and Zwietering (2006). The selection criterion for the test strain was defined as the highest possible heat resistance (D-value).
The stock solution used for the inoculum was made from a smear that was abraded from a previously produced and ripened cheese and inoculated on the surface with L. innocua at the beginning of the maturation process. After a ripening period of 4 weeks (on wooden shelves at 12 °C in 94% relative humidity) 7 g smear was abraded from the cheese surface (700 cm2). The smear was suspended in 30 mL sterile NaCl 3%, resulting in a cell count of 1.8 × 106 colony-forming units CFU/mL L. innocua. Two solutions have been diluted (NaCl 3%) from that stock solution: containing 4.26 log CFU/mL (Series A) and 6.26 log CFU/mL (Series B), respectively. Of each of these, respective solutions 0.6 mL were applied to the indicated area (25 cm2) on the surface of the wooden blocks and spread with an applicator, resulting in calculated inocula of 2.64 log CFU/cm2 (series A) and 4.64 log CFU/cm2 (series B), respectively. The inoculated blocks were stored at 12 °C and 94% relative humidity for 240 h. To avert desiccation of the blocks, all blocks were moistened after 144 h by spraying with sterile NaCl (3%) every 24 h. The blocks for the B series were inoculated just on the upper side. Blocks of the A series were inoculated on both sides, in order to allow an additional qualitative analysis using swabbing as a sampling method on the second surface. The cell counts of L. innocua on all blocks of both series were determined after 24, 72, 144 and 240 h, respectively. Samples for the sanitising runs were taken after 168, 192 and 216 h. No quantitative samples for surface cell counts on untreated blocks were determined for these sampling time points; in order to exclude eventual cross-contamination of the drilling system during the process of sampling the wood surface, estimated cell counts for these time points have been interpolated via linear regression among the cell counts at T = 144 and T = 240 h.
2.3 Sanitising heat treatment
The miniaturised steam cell followed the construction principles described by Imhof and Riva Scettrini (2015). Wooden blocks were placed into the steam cell sample cage (Fig. 1b). A full load consisted of 15 blocks. The cage was placed and locked onto a steam distributor (Fig. 1a), and the entire rack, including the sample cage and steam distributor, was covered and enclosed in a polyethylene bag and placed in the steam cells’ outer shell (Fig. 1d). A commercial steam cleaner produced steam continuously (Kärcher SC 1402, Kärcher AG, CH-8108 Dällikon, Switzerland).
a Steam cell with steam distributor showing steam inlet (e) and lower steam feeding (f); b loaded sample cage before the start, indicating the positions of the upper (h) and lower (g) temperature sensors relative to the loading of the steam cell; c closed system with upper steam feeding (i); d the overall system wrapped into a plastic bag before the start
Heat distribution and the finding of the system’s coldest spot were determined in test runs by a multi-channel data acquisition system (ALMEMO® 5690-2M, Ahlborn Mess- und Regelungstechnik GmbH, 83607 Holzkirchen, Germany). Temperatures were measured in 1-min intervals simultaneously at three points in three different levels with resistance-based Pt100 temperature sensors (Ahlborn Mess- und Regelungstechnik GmbH, 83607 Holzkirchen, Germany). The accuracy of the measurements in the range between 60 and 90 °C was <0.1 °C for each sensor.
The aim of this process was to create a temperature profile that guarantees a time span in which the temperature at the coldest spot of the steam cell showed temperatures higher than 70 °C for 15–20 min. The parametrical values were based on available heat resistance data (ICMSF 1996; Sörqvist 2003; van Asselt and Zwietering 2006) and should guarantee a sound safety margin for the described worst-case scenario for heat resistance (Sörqvist 2003; van Asselt and Zwietering 2006).
Dynamic temperature profiles (Table 1) V1 and V2 were determined from different runs in the evaluation process of a mobile steam cell for the sanitation of wooden boards. In sanitising runs with this mobile system, the steam production is switched off at the critical temperature value, and the system is kept sealed for 20 min. During these 20 min, the temperature steadily sinks from the critical temperature value to the final temperature value (Imhof and Riva Scettrini 2015). Temperature profiles V1 and V2 in the actual study follow and simulate the temperature development of these evaluation runs. To test an isothermal temperature profile, variant V3 consists of an isothermal course at 70 °C for 20 min (Table 1). Isothermal programmes are used in practice in more sophisticated, temperature-controlled types of steam cells. The efficacy of the heating process was determined by calculating the p-values of the various heating procedures (Wallhäusser 1978). The results were compared to an isothermal course at a reference temperature of 70 °C for 20 min: With D 70 °C = 0.52 min and z = 7.0 °C for L. monocytogenes (van Asselt and Zwietering 2006), a summed p-value of 20 min results in a total log reduction of 38.1.
Temperatures were controlled in the miniaturised system of this study by two Pt100 sensors that were each fixed 1 cm beyond and below the wood samples, respectively (Fig. 1b). On one wooden block, a Pt100 sensor for the reading of the core temperature was placed in a 4 cm-deep drill hole at the narrow side of the wood sample. The open hole around the sensor cable was sealed steam-tight with silicon paste to guarantee accurate temperature readings at the core. The temperature-recording interval was 15 s. The run procedure was as follows: The steam generator heated the system to the critical temperature value, which was measured at the lowest point of the loading, 1 cm below the wood blocks within the rack. Upon reaching this temperature, steam production was reduced to the according temperature programme, and the system was held, sealed, for at least 20 min before being opened, cooled and unloaded. The sample cage, including the heat-sanitised blocks, was stored in an applicable compartment to cool to room temperature.
2.4 Sampling the wood surface
Quantitative samples were taken by removing 2 mm of surface material from an inoculated area of 25 cm2 using a vertical drill press equipped with a Forstner bit (Craftomat Forstnerbohrer, Bauhaus AG, 68167 Mannheim, Germany) suited for drilling flat-bottomed holes of 32 mm diameter. Forstner bits have radial cutting edges to plane off the material at the bottom of the hole, producing 3–4 g wood shavings similar to a normal planing. The depth of the drill holes (2 mm) was chosen according to results from a study carried out at the Institut Technique Français des Fromages ITFF (Notz 2013), and cited summarised in the thesis by Mariani (2007), which revealed that the characteristic microbial flora used for cheese ripening colonised the wooden boards to a maximum depth of 2 mm. The inoculation area, designed as a triangle arrangement of three closely spaced circles of 32 mm diameter, including the central area in between the circles, comprises a total surface of 24.50 cm2. To avoid excessive heat production during the removal, which could destroy surviving bacterial cells, the drilling process was executed at the lowest possible speed (100 rpm), and the Forstner bit was preliminary cooled down for 10–12 s in liquid nitrogen. The sampling bags containing the wood blocks were firmly fixed in a machine vice, which was secured to the drill bed. This way, the drilling was carried out in a loss-free manner directly inside the sample bag (Fig. 2).
In addition, qualitative samples were taken from the second surface of series A blocks (inoculum 2.64 log CFU/cm2) by swabbing the inoculated area with sterilised and moisturised (NaCl 9 g/L) cotton dental rolls (10 x 38 mm, IVF-Hartmann AG, 8212 Neuhausen, Switzerland) for qualitative analyses.
2.5 Recovery of L. innocua in the wood blocks
The quantitative recovery of the test organism followed the protocol outlined in EN ISO 11290-2:1998/Amd.1:2004. The resulting wood shavings (3–4 g) were suspended in 80 g of ONE Broth Listeria Base (OXOID, Basingstoke, Hampshire, UK), containing ONE Broth—Listeria Supplement (OXOID, Basingstoke, Hampshire, UK) and homogenised in a stomacher blender (Biomerieux—AES Chemunex, 36172 Bruz, France) for 2 min. From this, a primary dilution 3 × 333 µL was plated directly onto three ALOA Agar plates (Biolife Italiana S.r.l., Monza, Italia); then, 100 µL of decimal dilutions in sterile NaCl 3% were equally plated onto ALOA-Agar (Biolife Italiana S.r.l., Monza, Italia), followed by incubation at 37 °C for 48 h.
The suspended wood shavings from the quantitative preparation were used for qualitative analyses by applying Agroscope’s in-house method for the detection of Listeria monocytogenes; this is based on ISO 11290-1:1996, with some validated modifications. (One-step enrichment for 24 h in ONEBroth® (OXOID), plating on ALOA-agar and confirmation of L. monocytogenes with an ACCUPROBE gene probe (Biomérieux).)
The suspended wood shavings were incubated at 30 °C for 24 h, plated onto ALOA-Agar (Biolife Italiana S.r.l., Monza, Italia) and incubated for 24 h at 37 °C.
If no colony was present on the quantitative agar plates, and if the corresponding qualitative analysis was positive, the threshold of detection (32 CFU/cm2) was used to perform statistical analysis.
The analyses of the cotton rolls of the swabbing samples followed the Agroscope in-house method for the detection of Listeria monocytogenes, which is based on ISO 11290-1:1996 and modified as described above. Cotton rolls were suspended in 80 g ONE Broth Listeria Base (OXOID, Basingstoke, Hampshire, UK) containing ONE Broth - Listeria Supplement (OXOID, Basingstoke, Hampshire, UK) and incubated at 30 °C for 24 h, plated onto ALOA-Agar (Biolife Italiana S.r.l., Monza, Italia) and incubated for 24 h at 37 °C.
3 Results and discussion
3.1 Selection of the test strain
Although the temperature programmes used in the heating process were based on a critical (minimal) temperature of 70 °C, the D-values of the evaluated test organisms were determined at 65 °C for practical reasons (the ratio of the volumes in the reaction tubes against time for cool-down to stop the heating would result in inaccurate results at 70 °C). All strains tested showed D65-values between 39 s and 46 s. The selected strain, Listeria innocua ILM 20870, resulted in a D-Value of D65 = 46 s, with corresponding values of logD70 = −0.119 min and z = 5.1 °C, respectively. This D-value is well within the range of D-values for L. monocytogenes and L. innocua available in the literature (Sörqvist 2003; van Asselt and Zwietering 2006). It therefore seemed justifiable to work with L. innocua ILM 20872, instead of L. monocytogenes.
3.2 Recovery of L. innocua from the wood blocks
There is no standardised sampling method for wood surfaces (Ismail et al. 2013). With this in mind, it was decided to use a destructive method (the removal of 2 mm surface material) to recover the totality of inoculated and grown Listeria innocua. Zangerl et al. (2010) used a similar method to produce shavings by planing the surface to a depth of 2 mm.
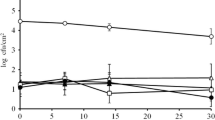
Although a substantial decrease (2 log CFU) in cell count occurred, L. innocua survived and was detectable on the surface of the wooden boards for the whole timespan of the experiment (240 h), independent of the concentration of L. innocua in the inoculum (Fig. 3).
Recovery of L. innocua from the wooden blocks (n = 3); coloured areas represent both qualitative detection and quantitative enumeration methods; dashed areas represent exclusively qualitative detection below the detection limit of the enumeration method. Time = 0 h: total inoculum, calculated value. Detection limit enumeration method = 32 CFU/cm2
The comparison of the surface swabbing method (sterilised and moisturised dental cotton rolls) and the abrasive (shavings) sampling method using the qualitative detection method resulted in identical findings. This shows the possibility of detecting even low concentrations of L. innocua <32 CFU/cm2 on wet wooden surfaces with the swabbing method. This swabbing method is successfully used for environmental hygienic monitoring in cheese dairies in Switzerland.
3.3 Efficacy of the heating processes
All three heating processes presented led to a Listeria-free hygienic status in the treated wood blocks (Table 2). The summed lethal rates in each process rose above the target value of 20 min at 70 °C, reaching exposure equivalents of 25 min and higher (Table 3), due to the inclusion of the contribution of lethal effects during heating and cooling. Calculated log-reduction values out of lethal rates and logD70 °C-values demonstrate that the proposed heating variants each reach reduction equivalents significantly higher than 10 log (Table 3).
In addition, all samples of the condensation water collected at the bottom of the steam cell were analysed as ‘negative’, which proves that the thermal process, even in the system’s coldest spot, was effective. These results confirm the results of an earlier-presented sanitising method for wooden boards via submersion in hot water (Zangerl et al. 2010).
As the heating process diminishes or eliminates the natural and functional flora on the boards, a certain risk of the propagation of L. monocytogenes might emerge, due to the absence of competitive flora in the case of infections during cheese maturation (Mariani et al. 2011). This lack of competitive flora certainly represents a high risk in restart situations during the first weeks after a clean-up. Empirical data for cheese dairies using steam cells on a regular basis for years demonstrate that the elimination of the risk of cross-contamination eventually outweighs the periodic reduction of the natural and functional flora on the boards. Imhof and Riva Scettrini (2015) have described two different types of steam cells and supplied data on product safety criteria over a span of 10 years. In both cases, the treatment of the wooden boards in steam cells led to the interruption of the quoted cheese dairies’ internal recontamination cycle, and the hygienic monitoring of indicator samples showed no positive results for L. monocytogenes, since the clean-up 10 years ago.
4 Conclusion
The presented steam-mediated heating process is an effective tool for the elimination of vegetative pathogens such as L. monocytogenes on wooden shelves or other working tools used in the production and maturation of cheese. The heating parameters of the standard heating process (temperature of the loading at the critical temperature point at 70 °C for at least 20 min) completely destroy Listeria innocua present at the surface and in the top 2 mm shavings of wooden boards. Two of the tested heating procedures are not of isothermal character, but reached top temperatures of 75–78 °C, even at the core of the wooden boards, before sinking back to 70 °C during the holding phase. The safety margin of the sanitising process can easily be increased (e.g., by switching off steam production at higher temperatures and/or prolonging the temperature holding time).
The tested swabbing sampling method showed results identical to the analyses of the abrasive method (shavings). With both sampling methods, it was possible to detect L. innocua on the surface of wet wooden blocks as low as <32 CFU/cm2. The tested swabbing method can therefore be used as an efficient sampling procedure for environmental indicator samples in listeria control.
Empirical data of cheese dairies that utilise a type of steam cell for the sanitation of wooden boards show that the system constitutes an important contribution to listeria control. The heat treatment process including appropriate verification data should be formulated as a Standard Operating Procedure (SOP) and be integrated as an additional measure into the operations’ quality management system.
References
Abrihami SH, Tall BD, Bruursema TJ, Epstein PS, Shah DB (1994) Bacterial adherence and viability on cutting board surfaces. J Food Safety 14:153–172. doi:10.1111/j.1745-4565.1994.tb00591.x
Büla CJ, Bille J, Glauser MP (1995) An epidemic of food-borne listeriosis in Western Switzerland: description of 57 cases involving adults. Clin Infect Dis 20:66–72
Carpentier B, Cerf O (2011) Review—persistence of Listeria monocytogenes in food industry equipment and premises. Int J Food Microbiol 145:1–8
Carpentier B, Chassaing D (2004) Interactions in biofilms between Listeria monocytogenes and resident microorganisms from food industry premises. Int J Food Microbiol 97:111–122
Doyle ME, Mazzotta AS, Wang T, Wiseman DW, Scott VN (2001) Heat resistance of Listeria monocytogenes. J Food Prot 64:410–429
EC (2004a) Regulation (EC) no 852/2004 of the European Parliament and of the council of 29 April 2004 on the hygiene of foodstuffs. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0852&rid=1. Accessed 4 July 2017
EC (2004b) Regulation (EC) No 1935/2004 of the European Parliament and of the council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R1935&rid=1. Accessed 4 July 2017
FDA (2014a) Clarification on using wood shelving in artisanal cheesemaking. US Food and Drug Administration. http://www.fda.gov/Food/NewsEvents/ConstituentUpdates/ucm400808.htm. Accessed 21 March 2017
FDA (2014b) US code of federal regulations, title 21, volume 2, revised as of April 1, 2014. US Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=110.40. Accessed 21 Mar 2017
ICMSF (1996) Microorganisms in food. Blackie Academic and Professional, London
Imhof R, Riva Scettrini P (2015) A simple steam cell for the sanitation of wooden boards used in the maturation of cheese. Agroscope, Institute for Food Sciences IFS, Bern. https://www-tmp.agroscope.admin.ch/agroscope/de/home/themen/lebensmittel/sicherheit/forschungsprojekte/lebensmittelsicherheit-milchprodukten/publikationen.html Accessed 12 Apr 2017
Ismail R, Aviat F, Michel V, Le Bayon I, Gay-Perret P, Kutnik M, Federighi M (2013) Methods for recovering microorganisms from solid surfaces used in the food industry: a review of the literature. Int J Environ Res Public Health 10:6169–6183
Mariani C (2007) Ecologie microbienne des biofilms présents à la surface des planches d’affinage en bois de l’AOC ‘Reblochon de Savoie’ et effet inhibiteur vis à vis de Listeria monocytogenes. (Wooden ripening shelves of PDO “Reblochon de Savoie”: microbial ecology and inhibitory effect against Listeria monocytogenes) Ecole Nationale Supérieure des Industries Agricoles et Alimentaires ENSIA (AgroParisTech). https://tel.archives-ouvertes.fr/file/index/docid/501220/filename/2007AGPT0089.pdf. Accessed 1 June 2017
Mariani C, Oulahal N, Chamba JF, Dubois-Brissonnet F, Notz E, Briandet R (2011) Inhibition of Listeria monocytogenes by resident biofilms present on wooden shelves used for cheese ripening. Food Control 22:1357–1362. doi:10.1016/j.foodcont.2011.02.012
Møretrø T, Langsrud S (2004) Listeria monocytogenes: biofilm formation and persistance in food-processing environments. Biofilms 1:107–121
Notz E (2013) Maîtrise de l’utilisation du bois comme auxiliaire technologie pour l’affinage des fromages—Synthèse des travaux ITFF – ACTIA 1997-2006 (Evaluation and mastering of microbiological risks in the use of wooden shelves in cheese maturation—synthesis of the results in the framework of the research study on the ACTIA-ITFF 1997-2006), Institut de l’élevage IDELE (The French Livestock Institute IDELE), Paris. http://idele.fr/?eID=cmis_download&oID=workspace://SpacesStore/cf1b9a28-31dc-4d87-9026-5dd9f8daf34b (Page 41, in French only). Accessed 19 May 2017
Peng S, Stephan R, Hummerjohann J, Blanco J, Zweifel C (2012) In vitro characterization of Shiga toxin-producing and non-Shiga toxin-producing Escherichia coli isolated from the raw milk cheese production process in respect of phenotypic traits and cheese-production relevant stresses. J Food Safety and Food Quality 63:136–141. doi:10.2376/0003-925X-63-136
Sörqvist S (2003) Heat resistance in liquids of Enterococcus spp., Listeria spp., Escherichia coli, Yersinia enterocolitica, Salmonella spp. and Campylobacter spp. Acta Vet Scand 44:1–19
van Asselt ED, Zwietering MH (2006) A systematic approach to determine global thermal inactivation parameters for various food pathogens. Int J Food Microbiol 107:73–82
Wallhäusser KH (1978) Sterilisation—Desinfektion—Konservierung. Georg Thieme Verlag, Stuttgart
Zangerl P, Matlschweiger C, Dillinger K, Eliskases-Lechner F (2010) Survival of Listeria monocytogenes after cleaning and sanitation of wooden shelves used for cheese ripening. Eur J Wood Wood Products 68:415–419. doi:10.1007/s00107-009-0381-6
Acknowledgements
The authors want to thank Emmanuelle Arias, for the cultivation of the bacterial strains on the surface of cheese and the support of the work in the BSL2 cheese maturation room, and Patrick Bischoff, for technical expertise and support in the development of the sampling process.
Author information
Authors and Affiliations
Contributions
René Imhof designed the study, developed the steam cell, operated the experimental runs, drafted the manuscript and interpreted the results. Livia Schwendimann characterised the strains of L. innocua, co-operated during the experimental runs and was responsible for the sampling and analytical tests. Patrizia Riva Scettrini co-developed the steam cell and delivered the parameters related to the practices for the experimental runs.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest: This research received no specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Funding
This research received no specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Imhof, R., Schwendimann, L. & Riva Scettrini, P. Sanitising wooden boards used for cheese maturation by means of a steam-mediated heating process. J Consum Prot Food Saf 12, 255–263 (2017). https://doi.org/10.1007/s00003-017-1114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-017-1114-0