Abstract
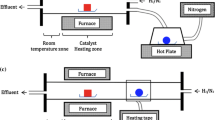
Chemical vapor deposition (CVD) is one of the best methods to grow graphene. In this way, there are many methods to produce graphene by liquid and solid precursor. In this research, we grew the graphene using ethanol and cyclohexane as precursors by some of these methods to obtain the best method to enter the precursor to the furnace. We compare the size of graphene domains by these methods and investigate the quality of their products. As the best method, we should keep inert gas into the precursor container to dilute the vapor of the precursor and heat the container to be sure that there is the vapor of precursor in it. Hydrogen should flow from a separate pipeline to the furnace. In this method, the rate of carbon precursor is measurable. Also, the results show that by increasing the growth time, graphene domains grow and become more significant. Also, with increasing the number of carbons in the structure of the precursor, the size of graphene will increase.


taken from the top surface of Cu by the first method using ethanol for 60 s. b An optical image takes from the back surface of Cu by the first method using ethanol for 60 s. c An optical image takes from the top surface of Cu by the first method using ethanol for 30 s. d An optical image takes from the back surface of Cu by the first method using ethanol for 30 s

taken from the top surface of Cu by the second method using ethanol for 2':20''. b The optical image took from the back surface of Cu by the second method using ethanol for 2':20''. c The optical image took from the top surface of Cu by the second method using ethanol for 2':30''. d The optical image took from the back surface of Cu by the second method using ethanol for 2':30''

taken from the top surface of Cu by the third method using ethanol for 140 s. b The optical image took from the back surface of Cu by the third method using ethanol for 150 s


taken from the top surface of Cu by the third method using cyclohexane for 140 s. b The optical image took from the back surface of Cu by the third method using cyclohexane for 150 s







Similar content being viewed by others
References
Zhang H et al (2012) Z-scan measurement of the nonlinear refractive index of graphene. Opt Lett 37(11):1856–1858
Balandin A et al (2008) Extremely high thermal conductivity of graphene: prospects for thermal management applications in silicon nanoelectronics. In: 2008 IEEE silicon nanoelectronics workshop. IEEE
Zhang P et al (2014) Fracture toughness of graphene. Nat Commun. https://doi.org/10.1038/ncomms4782
Zhong M et al (2016) Interface coupling in graphene/fluorographene heterostructure for high-performance graphene/silicon solar cells. Nano Energy 28:12–18
Wang X, Zhi L, Müllen K (2008) Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett 8(1):323–327
Stoller MD et al (2008) Graphene-based ultracapacitors. Nano Lett 8(10):3498–3502
Kim KS et al (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457(7230):706
Motallebipour MS, Karimi-Sabet J (2021) Graphenylene and inorganic graphenylene nanopores for gas-phase 4He/3He separation: kinetic and steady-state considerations. Phys Chem Chem Phys 23:14706–14715
Marsousi S et al (2019) Liquid–liquid extraction of calcium using ionic liquids in spiral microfluidics. Chem Eng J 356:492–505
Karbasi E et al (2017) Experimental and numerical study of air-gap membrane distillation (AGMD): novel AGMD module for Oxygen-18 stable isotope enrichment. Chem Eng J 322:667–678
Moradi R et al (2016) Air gap membrane distillation for enrichment of H218O isotopomers in natural water using poly(vinylidene fluoride) nanofibrous membrane. Chem Eng Process Process Intensif 100:26–36
Kusmartsev F et al (2015) Application of graphene within optoelectronic devices and transistors. In: Misra P (ed) Applied spectroscopy and the science of nanomaterials. Springer, Singapore, pp 191–221
Woltornist SJ et al (2013) Conductive thin films of pristine graphene by solvent interface trapping. ACS Nano 7(8):7062–7066
Tang L et al (2012) Bottom-up synthesis of large-scale graphene oxide nanosheets. J Mater Chem 22(12):5676–5683
Hofmann M et al (2015) Controlling the properties of graphene produced by electrochemical exfoliation. Nanotechnology 26(33):335607
Kang C, Jung DH, Lee JS (2015) Atmospheric pressure chemical vapor deposition of graphene using a liquid benzene precursor. J Nanosci Nanotechnol 15(11):9098–9103
Srivastava A et al (2010) Novel liquid precursor-based facile synthesis of large-area continuous, single, and few-layer graphene films. Chem Mater 22(11):3457–3461
Dong X et al (2011) Growth of large-sized graphene thin-films by liquid precursor-based chemical vapor deposition under atmospheric pressure. Carbon 49(11):3672–3678
Tu Z et al (2014) Controllable growth of 1–7 layers of graphene by chemical vapour deposition. Carbon 73:252–258
Wassei JK et al (2012) Chemical vapor deposition of graphene on copper from methane, ethane and propane: evidence for bilayer selectivity. Small 8(9):1415–1422
Wirtz C et al (2014) Growth optimisation of high quality graphene from ethene at low temperatures. Chem Phys Lett 595:192–196
Luo B et al (2013) Synthesis and morphology transformation of single-crystal graphene domains based on activated carbon dioxide by chemical vapor deposition. J Mater Chem C 1(17):2990–2995
Dou W-D, Yang Q, Lee C-S (2013) The effects of oxygen on controlling the number of carbon layers in the chemical vapor deposition of graphene on a nickel substrate. Nanotechnology 24(18):185603
Jang J et al (2015) Low-temperature-grown continuous graphene films from benzene by chemical vapor deposition at ambient pressure. Sci Rep. https://doi.org/10.1038/srep17955
Rao R, Weaver K, Maruyama B (2015) Atmospheric pressure growth and optimization of graphene using liquid-injection chemical vapor deposition. Mater Express 5(6):541–546
Guermoune A et al (2011) Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 49(13):4204–4210
Li Z et al (2011) Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 5(4):3385–3390
Zhang B et al (2012) Low-temperature chemical vapor deposition growth of graphene from toluene on electropolished copper foils. ACS Nano 6(3):2471–2476
Koosha N et al (2021) Improvement of synthesized graphene structure through various solvent liquids at low temperatures by chemical vapor deposition method. Mater Sci Eng B 274:115458
Mirzaei M et al (2020) Graphene growth with no intended carbon precursor feeding into the LPCVD process: causes, solutions, and effects. Nanotechnology 32(2):025604
Hedayat SM, Karimi-Sabet J, Shariaty-Niassar M (2017) Evolution effects of the copper surface morphology on the nucleation density and growth of graphene domains at different growth pressures. Appl Surf Sci 399:542–550
Bautista C, Mendoza D (2011) Multilayer graphene synthesized by CVD using liquid hexane as the carbon precursor. arXiv preprint arXiv:1109.1318
Kuten D et al (2020) Towards clean HSMG® graphene transfer. Mater Chem Phys 251:123161
Rummeli MH et al (2010) Direct low-temperature nanographene CVD synthesis over a dielectric insulator. ACS Nano 4(7):4206–4210
Nandamuri G, Roumimov S, Solanki R (2010) Chemical vapor deposition of graphene films. Nanotechnology 21(14):145604
Gong C-M, Li Z-R, Li X-Y (2012) Theoretical kinetic study of thermal decomposition of cyclohexane. Energy Fuels 26(5):2811–2820
Ferrari AC et al (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97(18):187401
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143(1–2):47–57
Eckmann A et al (2013) Raman study on defective graphene: effect of the excitation energy, type, and amount of defects. Phys Rev B 88(3):035426
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koosha, N., Karimi-Sabet, J., Moosavian, M.A. et al. The strategy of precursors entering furnace for graphene synthesis through the CVD technique. Graphene and 2D mater 7, 31–44 (2022). https://doi.org/10.1007/s41127-021-00046-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41127-021-00046-4