Abstract
With an ever-increasing demand for energy, there is a proportionate increase in energy storage devices, among which batteries hold the key to the energy transition. Globally, batteries constitute the fastest-growing energy storage technology that is playing a key role in the transport sector electrification leading to rising demand for LIBs. However, there is a substantial need for innovation that will help mitigate the environmental effects of the production and use of LIBs—such as energy use, mineral extraction, and chemical processing. The battery value chain can be seen as an exceptional sustainable value creation opportunity wherein sustainability depends in part on the ability to reuse and recycle batteries. A typical LIB battery serves in electric vehicles (EVs) for about 5–10 years and needs to be replaced when they reach ~ 20% capacity loss. At this stage, the fate of the battery follows one of the routes—disposal, reuse/repurpose/remanufacture (3R) or recycle. However, a major obstacle for car and battery manufacturers to invest in second life, or to otherwise take advantage of the reuse market, is that they in many cases do not have control over the batteries. On the other hand, recycling LIBs holds tremendous potential owing to the recirculation of materials i.e., closed-loop recycling needed for battery manufacturing promoting sustainability. This review will enable readers to devise processes that contribute to closing the loop of the EV LIBs value chain from an industrial perspective as well as critically understand the current state and future of battery recycling.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
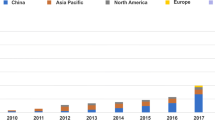
Lithium-ion batteries (LIBs) have revolutionized the electric vehicle (EV) industry due to their light weight, high energy density, long cycle life, compact size, low discharge rate, and wide temperature range for charging and discharging among others. Countries are moving rapidly towards transport electrification with China and Europe expected to be the biggest adaptors of EVs [1]. Figure 1a [2]shows that worldwide car sales share of EVs is projected to increase from 2 million in 2020 i.e., 2% sales share to 61% by 2040 (73 million units). In many developed countries, the share of EV sales is expected to be well over 80%. The global EV demand is also expected to be very strong, wherein by 2040 the number of EVs will increase by a factor of 60–70, compared to 2016 and will reach from 12 to 28% of global fleet [3]. Europe is expected to lead sales of EVs overtaking China around 2028 [2]. In Europe, Norway is leading from the front in the electrification of transport with almost 79% of new passenger cars being sold were EVs in 2022 and Fig. 1b shows an exponential rise in registered EVs in Norway in the last decade [4]. Since, there is a substantial cost difference between internal combustion engine vehicles (ICEVs) & EVs, various subsidies (direct/indirect) from the governments around the world are being formalized [5]. These cost comparisons between ICEVs and EVs are critical to understand as it will determine the future EV adaptation but falls outside the scope of this review.
The rapid transition towards EVs has resulted in increased global LIB production from 455 GWh in 2020 to 1,447 GWh in 2025, at a compounded annual growth rate (CAGR) of 26% [6]. An estimated 7.8 million tonnes per year of EV batteries is expected to reach end-of-life by 2040 [7]. This makes recycling of spent LIBs important for several factors—namely to reduce environmental impacts due to landfill (an option otherwise), secure robust supply of critical raw materials like lithium (Li), cobalt (Co), and nickel (Ni) whose supply are affected due to geopolitics, decrease the environmental costs associated with mining virgin resources and eventually closing the loop of EV LIBs value chain with an aim of a circular and sustainable economy.
Current commercial LIBs recycling technologies based on pyrometallurgy have high energy consumption and lead to the emission of harmful gases such as NOx & SOx which need additional scrubbing systems, thereby decreasing techno-economic viability of such processes [8, 9]. The product is mostly an alloy that is further processed by hydrometallurgical routes; however Li and aluminium (Al) are lost in the slag. Moreover, these pyrometallurgical technologies are capital intensive and become financially inviable when Ni and Co are less than 30% in the feed [10]. On the other hand, LIBs recycling processes based on hydrometallurgy incorporate various pre-treatment steps for initial separation followed by chemical recovery employing co-precipitation, solvent extraction, ion exchange, and sol–gel methods among others. Hydrometallurgical technologies in spite being complex and operationally intensive are gaining interest as they have superior value recoveries, lower operational temperature, lower gaseous emissions, etc. as compared to pyrometallurgical technologies [11, 12].
Such processes have been the focus of recent reviews which detail on existing commercial technologies as well as laboratory-based processes within research and development. Chen et al. [13] discussed EV LIBs recycling technologies based on pyrometallurgy, hydrometallurgy, and direct recycling. They also touched upon the challenges related to each process along with the process description from commercial applications. Harper et al. [14] in their review on EV LIBs recycling touched upon various recycling processes, limitations, and potential areas of improvement. Li et al. [15] studied hydrometallurgical treatment of spent LIBs including various unit operations like leaching, solvent extraction, and precipitation. They also discussed the pretreatment processes and resynthesis of cathode active material (CAM). Pineger et al. [16] in their review article discussed the various spent LIBs recycling processes which are not commercialized. It should be noted that their review suggested that most of the upcoming processes are based on hydrometallurgy. Earlier review works have discussed the state-of-the-art recycling processes mainly based on metallurgical route but little emphasis on closed loop recycling process which is essentially using the values from spent LIBs to manufacture new LIBs or alternative materials. In this review, we have critically assessed hydrometallurgy-based unit operations that can be part of closed-loop recycling routes which may further be scaled up industrially and this scope is illustrated in Fig. 2. We have broadly categorized treatment options into pre-treatment, hydrometallurgical recycling, and direct recycling. While the pre-treatment section involves discussion on battery discharging, disassembly and shredding, the various hydrometallurgical unit operations are explained with examples from relevant literature in the succeeding section. Leaching, solvent extraction, and chemical precipitation are in focus and are integrated into closed-loop routes that have been reported in the literature. A discussion on an upcoming promising recycling technology called direct recycling follows. This review critically discusses the importance of closing the loop of LIBs value chain on the global level and concludes with a future outlook of the state of EV LIBs recycling.
Present and Future Trends in EV LIBs
LIBs were first commercialized by Sony in 1991 [17]. A typical battery pack contains modules (8 to 48 per pack) which in turn contain cells (4 to 444 per module) that may vary in design such as cylindrical, prismatic, pouch for different manufacturers. However, the basic components of a LIB cell are identical: comprising cathode, anode, electrolyte, separator, binder, and current collectors as shown in Fig. 3 [18, 19]. The initial LIB cell was based on a lithium cobalt oxide (LCO) chemistry cathode which due to low energy density, inferior safety, short life span, and high cost has been replaced by the layered lithium nickel manganese cobalt oxide (NMC) and lithium nickel cobalt aluminum oxide (NCA) cathodes [20]. These are known to offer a high energy density, thus higher range, extended life span with cycles of 1000–2000 and 500 respectively, and lower cost as compared to LCO [21]. Currently, NMC cathodes are the choice for EV LIBs and the market trend for NMC is moving from low Ni to high Ni/low Co i.e., from NMC 111 to NMC 811 (where the numbers refer to mole ratios of Ni, manganese (Mn) and Co) and finally lithium nickel oxide (LNO) [22, 23]. The energy density and capacity for the NMC cathodes are in the range of 592–740 Wh kg−1 and 160–200 mAh g−1 respectively, with the highest values for NMC 811 cathode materials [23, 24]. The high costs of cobalt as well as future supply chain challenges coupled with better performance make these candidates the future choice. Table 1 gives a brief overview of the cost of various cathode active material which includes both metal cost (USD Kg−1) as well production costs & profit margins (PCPMs) (USD Kg−1) [25]. The expected LIBs cathode chemistry trend in Europe is illustrated in Fig. 4. It may be mentioned that eventually, lithium iron phosphate (LFP) is going to dominate the future LIBs market, especially in China. LFP chemistry offers a safe operation at higher temperatures along with specific energy and capacity of 580 WhKg−1 and 165 mAhg−1, respectively [23, 24].
Future trends of cathode chemistries in Europe [22]
Graphite and silicon anodes are commonly used in LIBs, [26, 27] but graphite anodes are commercially favoured due to their attractive price, abundant and superior life span [28]. Al and copper (Cu) are used as the current collector for cathode and anode, respectively [29]. They enable the flow of electrons from external circuits. LIBs use lithium salts such as lithium hexafluorophosphate (LiPF6), lithium perchlorate (LiClO4), lithium hexafluoroarsenate (LiAsF6), and lithium triflate (LiCF3SO3) as electrolytes within a non-aqueous medium with a fraction of equivalent lithium salt (ionic liquid) and plays the role of shuttling the mobile ionic species between cathode and anode [30]. There has been a continuous effort on developing a sustainable electrolyte for lithium-ion batteries. Separators are used to separate the anode and cathode for safety purposes. Polyolefin polymers are used widely as separators [31].
An overview of present and future trends of cathode, anode, electrolyte, current collector and separator is given in Table 2.
Recycling
When the EV battery capacity falls below 80% they are discarded and refurbished for second life usage also known as secondary batteries and are generally used as electricity storage in a power grid or solar electricity plant [39, 40]. Recently Fujita et al. [21] has written a comprehensive review focusing on the 3R-reuse, repurpose and recycling strategies for spent EV LIBs from automobiles. Lih et al. [41] in their studies presented the technology challenges, cost issues and analyses, and optimal business model for the second-use applications of retired Li-ion battery packs. Secondary batteries although looks promising but comes with their own set of challenges including cost of refurbishment, fire hazard linked with spent battery storage etc. and eventually these secondary batteries will wear off and will have to be recycled. Hence, developing a robust recycling technology for treating the spent LIBs is critical. Figure 5 represents a general scheme for spent EV LIBs for a typical recycling route which includes major steps of pre-treatments (Sect. 3.1) followed by hydrometallurgical unit processes (Sect. 3.2) like leaching, solvent extraction & chemical precipitation.
Pre-treatment Processes
Spent LIBs Discharging
Spent EV LIBs might have some residual charge which can be a potential safety hazard, thus making discharging spent batteries the first step in the pre-treatment process. Such batteries might have a residual voltage of 0.5–5 V and with discharging this should be brought down to 0 V [42]. Discharging techniques can be broadly classified into physical discharging, chemical discharging, and cryogenic freezing [43, 44]. Physical discharging includes attaching a battery to an external discharger, but this process is not feasible on the commercial scale. Chemical discharging is the most frequent technique used wherein various discharging media naming NaCl, fenton solution, Na2SO4, and ZnSO4, etc. are used [44,46,47]. He et al. analysed NaCl solution that works efficiently for discharging and further stated that 5% NaCl will be optimum for complete discharging [48]. Cryogenic freezing is also a potential option for discharging but was not found to be a preferable commercial option for discharging due to high costs related to installation of equipments [44].
Disassembly and Shredding
After discharging, spent LIBs are safe to operate with and are taken for disassembly and shredding with the primary aim to separate CAM from the battery pack to have a superior recovery. Many authors have reported manual methods for dismantling along with the removal of steel and plastic cases, cathodes, and anodes [49,50,51]. However, manual dismantling isn’t desirable for industrial applications especially for large volumes when safety and cost of operation will become significant. Mechanical dismantling including crushing and sieving is preferred due to its economic feasibility for industrial applications. In the mechanical crushing method as reported by Xiao et al. and Wang et al. [52, 53], discharged spent LIBs were firstly crushed under an inert environment which prevents combustion and explosion hazards, but resulted in particles of different sizes and distributions [54]. The experimental results of Martínez et al. depicted that Cu and Al were concentrated employing statistical entropy-based sieving for successful separation of the battery components [55]. The mechanical dismantling, though looks promising, comes with disadvantages as it negatively influences surface properties of battery electrodes for hydrometallurgical processing. This happens due to deposition of organic layers over the cathode powder caused by the decomposition of battery electrolyte and binder during crushing [56].
Another challenge due to the above-described mechanical dismantling is mixed anode and cathode particles which are coated with organic layers. Flotation can emerge as an effective technique for the separation of CAM from graphite (anode constituent) [57, 58]. Non-polar graphite is hydrophobic; thus, it sticks to the froth and can be separated from hydrophilic polar CAM which is removed from tailings. However, due to the organic layer on the particles, the wettability difference between CAM and graphite is compromised making them difficult to be separated by flotation [58]. He et al. [59] studied this problem and reported Fenton solution assisted flotation for the removal of this organic layer from the surface of the particles and by adjusting the solid–liquid ratio to 1:120 for Fe2+ and H2O2, the obtained recovery rate for graphite was reported to be 98.9% [59]. Shin et al. studied the froth flotation for an NMC cathode of EV pouch cell employing kerosene as collector and methyl isobutyl carbinol as frother and achieved CAM content of 90.05% in the tailings [60].
To ease hydrometallurgical recovery downstream, it is important to remove polyvinylidene fluoride (PVDF) the binder between foil and electrode material [61, 62]. According to literature, PVDF dissolves completely in N-methyl-2-pyrrolidone (NMP), hence the process of ultrasonication with NMP or NMP dissolution have gained interest [63]. NMP dissolution has its disadvantages such as high toxicity and increased operational costs which hinders its application on industrial scale [64].
Robotics along with artificial intelligence is making pre-treatment processes fully automated essentially addressing challenges like process safety concerns, scale of operations and operational savings in manual labour. Tan et al. [65]. in their work has conceptualized the disassembly procedure in an attempt to reduce the overall number of disassembly steps using robotics. Wegener et al. [66] also worked on developing a robot-assisted disassembling system of EV batteries and investigated the development of a planned disassembly system and workstation for the hybrid car Audi Q5 [67]. A futuristic disassembling was proposed by Zhou et al. [68] which consists of three systems namely battery information acquisition system, battery removal system, and battery classification system, claiming to decrease the disassembling time by 80–90% compared with manual disassembling. The future of battery pack disassembly will require more such automation processes that are able to cater to large volumes of spent LIBs.
Thermal Pre-treatment of Spent LIBs
Thermal pre-treatment of spent LIBs significantly improves value recovery of CAM as compared to the untreated spent LIBs [69] and aids the separation of electrode material from the organic binder, which require further off gas treatment [70, 71]. Bi et al. in their work proposed a low-temperature thermal pre-treatment employing vacuum pyrolysis (VP) resulting in thermal decomposition of binder and organic solvent. This aids in peeling off electrode material from the current collectors reducing equipment and energy cost [72]. Sun and Qiu reported detachment of CAM from Al foil and concluded that the pyrolysis temperature of 873 K resulted in complete separation [73]. Zhang et al. proposed an ultrasonic-assisted flotation followed by pyrolysis for the separation of (graphite) and CAM which increased recovery from 74.6% to 96.8% when compared with simple vacuum pyrolysis [74]. Incineration was tested in the work of Vieceli et al. as an effective pre-treatment method and their results demonstrated that incineration promoted a carbothermic reduction of the metals and even using low concentrations of sulfuric acid, higher leaching efficiencies of Co, Ni, Mn and Li were attained [75]. Pyrolysis is generally preferred over incineration for the separation of the cathode active material due to lower carbon dioxide emissions, although incineration may be cost-effective as reported by Lombardo et al. [69].
After thermal pre-treatment of spent LIBs, the NMC values forms a complex alloy leaving lithium which can be selectively leached. Träger et al. [76] reported the direct vacuum evaporation at high temperature and selective entrained carbon gas evaporation methods to separate lithium from spent LIBs. Higuchi et al. [77] in their process successfully separated lithium using Na2S2O8 as an oxidant from mixed cathodes of spent LIBs. Hu et al. reported a powerful reduction roasting technique for the recovery of lithium as lithium carbonate through reduction roasting followed by water leaching. The process successfully separated 99% Ni, Co and Mn and 84.5% Li under heating at ambient temperatures and water leaching conditions of 10:1 solid liquid ratio. Their method opened pathways for efficient separation of lithium at an industrial scale [78].
In this section, we have highlighted some of the important aspects of pre-treatment of spent LIBs. For detailed reviews, the reader is referred to the recent works of Zhang et al. [79], Kim et al. [80] and Yu et al. [81].
Hydrometallurgical Unit Processes
The spent LIBs after discharging, disassembly & physical separation form a black powder (known as black mass) which then undergoes further hydrometallurgical processing involving various chemical processes with major steps of extraction, concentration, and recovery of metal values. The extraction step is mainly transferring value from LIBs black powder to aqueous medium achieved by leaching unit operation which can employ reagents like inorganic acids, organic acids, or bases. The biggest advantage of the leaching is the possibility of complete value transfer from black powder to the leached solution provided operational conditions like temperature, stirring, residence time & solid to liquid (black powder to leaching solution) ratio are optimized. Leaching is followed by concentration steps such as solvent extraction and ion exchange with a focus on the separation of targeted value from the leachate solution with higher concentration. High-value recovery & high selectivity are the major advantages, but this comes with high operational cost and complex operations. Finally, the hydrometallurgical process ends with recovery unit operations like chemical precipitation, electrowinning etc. In LIBs recycling industries, supersaturation is generated by modifying the solution pH leading to precipitation and/or agglomeration to recover individual value (selective precipitation) or to collectively recover multiple values (co-precipitation). The following sections will discuss the key hydrometallurgical processes like leaching, solvent extraction and chemical precipitation in detail. This review covers hydrometallurgical processes on recycling spent LIBs employed in various applications with focus on the fundamental recycling principles. The recycling of EV battery packs differs from portable devices (mobile phones, laptops etc.) in the way they are pre-treated and prepared for hydrometallurgical processing e.g. discharging and dismantling the big battery packs can cause electrocuting; short circuiting resulting in temperature rise, eventually causing thermal runaway, releasing toxic gases (HF among others); extensive sorting for direct recycling of EV LIBs, to name a few [14]. However, once the black mass is prepared, the hydrometallurgical unit operations (discussed in this article) form the cornerstone for further processing.
Leaching
Leaching is a process of metal extraction from black mass with acids or bases. The appropriate conditions for leaching a specific metal value can be determined through thermodynamics and respective phase diagram. Black mass leaching studies have been done with inorganic acids like sulphuric acid (H2SO4) [82], nitric acid (HNO3) [83], hydrochloric acid (HCl) [84]; organic acids like citric acid(C6H8O7) [85], oxalic acid (C2H2O4) [86], malic acid (C4H6O5) [87] and lactic acid [88]; ammonia leaching [89] and bioleaching which explores the use of microorganisms like fungi, acidophilic bacteria, and chemolithotroph as leaching agents [90]. A typical industrial leaching set up is shown in Fig. 6 which consists of an autoclave reactor for high pressure and temperature leaching followed by a filter press for solid and leachate separation followed by a leachate storage tank.
Significant portion of Mn and Co in black mass is in the higher oxidation state (Mn4+, Mn3+ & Co3+ ions) that are not readily leachable due to higher redox potential of 1.51 V and 1.84 V respectively. Thus, using a reducing agent like hydrogen peroxide (H2O2) or sodium bisulfite (NaHSO3) increases the leaching efficiency and promotes the kinetics [91, 92]. The reduction mechanism of Co3+ to Co.2+ with H2O2 is given as follows: [93, 94]
Mineral Acid Leaching
Sulfuric Acid (H2SO4) One of the most widely researched and industrially preferred leaching agents for black mass is sulphuric acid. Meshram et al. studied the use of H2SO4 without any reducing agent [8] in a bench scale study of mixed cathode type spent LIBs from laptops wherein they were successful in leaching out 93% Li, 66% Co, 96% Ni, and 50% Mn over 240 min of leaching time at 950C using 1 M H2SO4 [8]. The lower leaching efficiency of Co and Mn is because the Co3+, and Mn.4+ are not readily dissolved in H2SO4. Shin et al. reported in their laboratory scale studies, that with the use of 15% H2O2 as a reducing agent, almost entire Co and Li can be extracted with reduced leaching time [95]. The equations representing the sulphuric acid leaching in the presence of different cathode chemistries are shown below: [96]
Zheng et al. recycled mixed spent LIBs (from portable devices and EVs) and were able to achieve leaching efficiencies of 91.1, 94.7, 94.1 and 90.9% for Li, Co, Ni, and Mn respectively by using 2.5 M H2SO4 with 5% H2O2 at 60.0C for 1 h [96]. A recent study by Zhao et al. employed the use of ethanol as the reducing agent in sulfuric acid systems for LCO type spent LIBs (from cell phones) in a 500 ml laboratory scale and their results showed that organic reductants can also be used for recycling LIBs with efficiencies of more than 99% for Li and Co [97]. The reactions associated with this process are shown below: [97]
Chen et al. studied cellulose, sucrose, and glucose as organic reducing agents with sulfuric acid for LCO electrodes in a laboratory based study which showed that glucose and sucrose can be successfully used as reducing agents, whereas Co leaching efficiency was not high in cellulose and sulfuric acid system [98].
Hydrochloric Acid (HCl).
Zhang et al. studied the leaching of LCO type LIBs (from portable devices) with 4 M HCl at 500 ml laboratory scale and successfully leached around 99% of Li and Co [99]. HCl is a very strong acid, and it has been widely reported that additional reducing agent does not impact leaching. More recently, Wang et al. studied optimum conditions for leaching NMC LIBs using 4 M HCl at 80 °C for 1 h at 500 ml volume scale, achieving efficiencies of ~ 99% for Li, Ni, Mn and Co [100]. The leaching equations for different cathode chemistries are shown below:
Nitric Acid (HNO3) Leaching of spent LIBs (LiMnO2 cathode) using 2 M nitric acid at 800C for 2 h (lab scale study) showed leaching efficiencies of 100% and 95% of Li and Mn, respectively, in a study by Castillo et al. [101]. The leaching reaction was reported to be the following:
Lee et al. [102] studied leaching of LiCoO2 cathode (from cell phones) at 750C for 30 min at solid/liquid ratio of 10 g/L using 1 M HNO3(leaching agent) and 1.7 M H2O2 as reducing agent and achieved an overall leaching efficiency of 95%. They also reported that with 1 M HNO3 without reducing agent, the leaching efficiency was 40% for Co and 75% for Li.
During our review we found that extensive work has been done in the field of inorganic acid leaching for LIBs recycling, but more studies are dedicated to H2SO4 leaching compared to HCl and HNO3. This is attributed to its low cost, high leaching efficiency and easy handling, making it preferable for industrial use.
Organic Acid Leaching Although, inorganic acids described above show excellent leaching efficiencies, there are several environmental challenges namely liberation of toxic NOX and SOX gases along with the acidic effluent which must be treated in a dedicated effluent treatment facility (ETP). To address these challenges, researchers are focussing on the use of organic leaching agents, which can be divided into various groups based on different number of carboxylic groups like monocarboxylic (acetic acid, lactic acid, formic acid), dicarboxylic (ascorbic acid, maleic acid, oxalic acid, tartaric acid), and tricarboxylic (citric acid) to name a few [103]. The organic acids are generally used with reducing agents like H2O2, ascorbic acid etc. to ensure higher leaching efficiencies.
Lactic acid and acetic acid dissociate completely in one step and are regarded as weak acids. Golmohammadzadeh et al. reported acetic acid leaching of consumed LCO cathode LIBs (from laptops) at a laboratory scale of 500 ml, recovering 30% Co and 75% Li with an optimized solid–liquid ratio of 30 g/L [94]. Li et al. experimented on lactic acid to recycle NMC type LIBs in an optimized solid–liquid ratio of 20 g/L with 0.5 vol% H2O2 and 1.5 M lactic acid leaching around 98% Ni, 97% Li, 98% Co and 98.4% Mn in 20 min at 100 ml scale [88]. Employing 1.25 M ascorbic acid with solid liquid ratio (s/L) of 25 g/L for 20 min at 700C during leaching of LCO type spent LIBs (from cell phones and laptops), Li et al. reported 94% Co and 98% Li recovery, and their results showed that ascorbic acid acted as a reducing agent [49]. Amongst all organic acids, oxalic acid has shown the highest leaching efficiency as reported in various works, owing to the higher activity of oxalic acid to liberate hydrogen ions [104]. Exposing the pre-treated cathode material to oxalic acid leaching, the oxidation state of cobalt oxide was reduced immediately as reported by Zeng et al. [86]. He et al. reported leaching of spent NMC type LIBs from various portable devices at laboratory scale using L-tartaric acid with 4% H2O2. Their results showed leaching efficiencies of 99% Ni, 98% Co, 99% Mn, and 99% Li [48]. According to the literature, temperature for leaching LIBs in presence of citric acid was 90 °C for recovering cobalt and lithium [105]. An organic acid recently reported by Pant et al. [51] was derived from a citrus fruit juice with a composition of ascorbic, citric, and malic acids. It resulted in removal of the binder without the use of toxic NMP solvent and provided a higher leaching efficiency from LCO type spent LIBs (from mobile phones).
Organic acid leaching is a greener alternative as compared to conventional mineral acids but comes with various challenges like lower leaching capacities, operations at low solid liquid ratios, slower leaching kinetics and higher costs which makes organic acid leaching unattractive at industrial scale.
Ammonia Leaching For the selective leaching of the values from spent LIBs, ammonia leaching has gained attention owing to its low toxicity, low cost and recyclability as compared to other leaching agents [89]. According to literature, ammonia reagent form complex compounds with Co, Li, and Ni while Al, Fe, and Mn are left in the residue [89]. Wang et al. compared the leaching efficiencies of various ammonia solutions for NCM cathode LIBs and emphasized the use of reducing agents in addition to increase the leaching efficiency. Their results reported leaching efficiencies for various ammonium salt solutions with different reductants in order of (NH4)2SO3 > Na2SO3 ≈ Na2S2O3 > Na2HPO3 [106]. Usually, the ammonia leaching process employs a two-step leaching in which the residue from first leaching step is used as raw material for second one to improve the leaching efficiency [107, 108]. A two stage leaching process was employed in a study by Zheng et al. and efficiencies increased substantially for Ni, Li and Co under leaching conditions of 4 M NH3, 1.5 M (NH4)2SO4 and 0.5 M Na2SO3 at 353 K, 10 g/L pulp density and 300 min from spent NMC cathode scrap in a laboratory based study. The authors reported leaching efficiencies of 89.8%, 4.3%, 80.7% and 95.3% for Ni, Mn, Co and Li, respectively [108]. These efficiencies can be increased further with the use of (NH4)2CO3 in leaching solution. Another laboratory study by Wang et al. on two feed materials from commercial as well as spent NCM type LIBs reported the efficiency increase from 85.3%, 79.1%, 86.4% to 97.3%, 98.4% and 99.4% for Ni, Li and Co, respectively, under leaching conditions of 4 M NH3, 1 M (NH4)2CO3, and 0.3 M Na2SO3 at 80 °C with a reaction time of 300 min [109]. Ma et al. also studied NCM type spent LIBs leaching with ammonia at a laboratory scale. They found that 97.7% Ni and 99.1% Co can be leached with 3 M NH3·H2O, 1.5 M (NH4)2SO3, at s/L ratio of 1:20 g/L at 353 K for 120 min [110].
There are multiple challenges with thermodynamically favoured ammonia leaching such as poor kinetics, formation of stable metal ammonia complexes which lowers recovery of metals through selective precipitation and solvent extraction and formation of harmful NH3 gas [111].
Bioleaching Bioleaching explores the use of microorganisms such as fungi, acidophilic bacteria, and chemolithotrophic as leaching agents [112]. These processes can be an attractive alternative as they are cost-effective, less energy-intensive, and technically less complicated [112]. These microorganisms are classified as chemolithotrophs and chemoorganotrophs, where the former thrive at low pH (2 or below) using the inorganic compounds as an energy source (acidophiles) while the latter utilize organic compounds as the energy source, such as fungi and cyanogenic microbes [90]. The mechanism of bioleaching can be named as contact and non-contact (also referred to as direct and indirect respectively) [90, 113]. As the name suggests, these mechanisms refer to the existence and non-existence of physical interactions between the microorganisms and the cathode material during the leaching process, respectively [90]. There are different approaches to perform the bioleaching experiments. The one-step approach deals with unifying microbial culturing, growth, and metal leaching together, but it has less efficiency due to the presence of metal ions in microbial growth [114]. In a two-step process, the microbes are allowed to grow separately and then the solid cathode material is added to initiate the leaching process. Thus, yield in this process is higher than the one-step approach [114]. To further increase the recovery yield, one can use spent medium leaching in which the cultured microbes, producing metabolites, are first centrifuged and then filtered to attain a cell-free medium. Solid materials are then added to these biogenically produced metabolites to carry out the leaching process [112]. This approach benefits from separation of biological and chemical steps necessary for the bioleaching process allowing harsher operational conditions in the chemical step to enhance recovery. However, this comes with high cost [112]. Acidithiobacillus ferrooxidans was used to produce biogenic sulfuric acid and ferric ions, into which NMC black mass was added and leaching was carried out for 72 h with three replenishing cycles, achieving efficiencies of 89%, 90%, 92% and 82% for Li, Ni, Mn and Co respectively [115]. Mixed culture (Acidithiobacillus thiooxidans & Leptospirillum ferriphilum) and mixed energy source (elemental sulfur: pyrite at 1:1 by weight) was also studied for bioleaching of LMO, LFP, and NMC cathode (from EVs) at laboratory scale. The study reported more than 95% extraction efficiency of Li, Ni, Mn, and Co [63].
Bioleaching, although theoretically exciting, comes with several limitations like slow kinetics, low leaching efficiencies, extensive pre-treatment, low pulp density and high operational and capital costs which makes the process industrially unattractive.
Table 3 shows an overview of the various leaching conditions that have been employed in the literature for metal extraction from different LIB cathode chemistries.
Solvent Extraction
Solvent extraction is based on the uneven distribution of solute between organic and aqueous phases to separate specific metal ions. The feed solution is brought in contact with an organic solvent in a mixer settler followed by the transfer of solute to the organic phase due to higher affinity along with some of the impurities as shown in Fig. 7. The two phases i.e., extract phase (organic loaded phase) and raffinate (aqueous phase) are separated by density difference. The scrubbing stage is designed for removing impurities from extract phase using weak acid/weak base in the subsequent mixer settler due to the density difference. The organic solution is further treated for solvent regeneration & solute is recovered by stripping. The regenerated solvent is recycled, and the stripping solution is treated for recovery of solute by chemical precipitation, ion exchange, etc. The McCabe–Thiele diagram is used to determine the required number of stages for extraction and stripping. The effectiveness of solvent extraction can be determined by pH50—equilibrium pH value when 50% of the metal ions are extracted and the separation factor (β) [124] which can be defined as the efficiency of the separation of solute from the other impurities present in the solution.
DM1 = Distribution factor of solute (metal ion), DM = Distribution factor of other solute (metal ions) in the solution.
Regarding β, generally, β > 10 means a possible separation, β > 100 indicates good separation, and β > 1000 suggests excellent separation[125]. The separation factor (β) on the other hand depends on the distribution factor (D) [126] which can be defined as follows:
[M]initial = metal ion concentration in the feed solution, [M]aq = metal ion concentration in the aqueous solution (raffinate) after solvent extraction.
A higher value of D indicates superior extraction yield of the metal ions from the solution using a solvent.
Many extractants namely di(2-Ethylhexyl) phosphoric acid (D2EHPA), diethylhexyl phosphoric acid (DEHPA), bis(2,4,4-trimethyl-pentyl) phosphinic acid (Cyanex 272), trioctylamine (TOA), and 2-Ethylhexyl phosphonic acid mono-2-Ethylhexyl ester (PC-88A) have been used to recover the valuable metals from spent LIBs leaching solution. Wesselborg et al. [127] studied the Li recovery from LIBs leachate at laboratory scale of 50 ml and the organic phase was prepared by mixing 80% TBP as extractant with 20% kerosene as diluent. In single-stage experiments with ratio of organic to aqueous phase [R(O/A)] = 1, 87.7% Li was extracted, and the loaded organic phase was scrubbed with 1 M LiCl + 2 M AlCl3 solution. Wang et al. [128] did laboratory studies which focus on Co extraction in the leaching liquor of spent LIBs with D2EHPA to separate Cu & Mn from the leached solution and PC-88A was used to separate Co with recovery of 80.13%. Other work at laboratory scale focused on the Co extraction can be followed in [117, 128,129,130]. Joo et al. [131] investigated the selective recovery of Ni from NMC 111 cathode material from spent LIBs employing a combination of Versatic 10 acid and LIX 84-I as solvents at 500 ml scale. The study showed that the optimum pH to extract Ni was 5 and the McCabe–Thiele diagram showed that a three-stage system was needed for the extraction of 100% Ni. Chen et al. [82] studied solvent extraction at laboratory scale for selective Mn recovery with cobalt loaded D2EHPA (Co-D2EHPA) and the Mn extraction of over 99% was achieved using two theoretical counter current stages under extraction time 5 min, pH 3.5, 15% Co-D2EHPA, and R(O:A) 1:1. Some of the other works on Mn recovery with solvent extraction can be followed in references [82, 124, 132]. Solvent extraction is used extensively in various industries but challenges such as high cost of the solvent, co-extraction, and complex operations prevail. There is a need to focus on future research for developing cheap, green and stable (across pH range) solvents. Table 4 lists some of the important research work done on metal recovery by solvent extraction.
Precipitation
Another important unit operation used in combination with solvent extraction or on its own after leaching is chemical precipitation that can be defined as a reaction between soluble metallic ions with the precipitating agent to recover them as insoluble compounds [48]. Supersaturation is the driving force behind precipitation which is essentially based on the difference between solute concentration and equilibrium concentration and dictates nucleation and growth events that characterize the resultant crystals. The crystal properties are further modified through secondary processes such as agglomeration and aging leading to final precipitation. Precipitation is used effectively in recovering values from the leached solution of spent LIBs as metal salts have pH dependent solubilities. Cu, Al, and Fe are usually precipitated at low pH (< 5) followed by co-precipitating Ni, Mn and Co at moderate pH (≈10) and finally Li is recovered at pH (~ 12) which forms the basis of selective precipitation. Alternatively, in some processes, Li may be precipitated in the beginning through carbonation while the other values are recovered as mentioned above. In a co-precipitation type recovery, the focus is on simultaneous recovery of multiple metal ions (Ni, Mn & Co) which can be used effectively to resynthsize cathode active material and close the recycling loop. Li precipitate is further mixed with the co-precipitated precursor and calcined to regenerate the cathode active material LiNixMnyCozO2 [142]. The ratio of Ni, Mn & Co can be modified by the addition of chemical salts like nickel sulphate hexahydrate, manganese sulphate monohydrate, and cobalt sulphate [143]. Both selective precipitation and co precipitation scheme can be followed in Figs. 8 and 9 respectively.
Zhu et al. [144] worked on recycling Co and Li from the spent LIBs leachate in which Co was precipitated by adding ammonium oxalate ((NH4)2C2O4) and Li was precipitated using sodium carbonate (Na2CO3) with recovery of 94.7% of Co and 71.0% of Li. Meshram et al. [145] focused on recovering Li, Co, Ni, and Mn from the spent LIBs leach liquor, > 98% Co was recovered as cobalt oxalate (CoC2O4.2H2O) by precipitation with oxalic acid followed by the precipitation of MnCO3, NiCO3, and Li2CO3 by the addition of sodium carbonate at pH of 7.5, 9 & 14, respectively. Pegoretti et al. [146] recovered Co as cobalt hydroxide with the addition of KOH to the LIBs leaching solution. There is substantial work done by Wang et al. [100] to recover various values from the LIBs leached solution, first, Mn was precipitated by KMnO4 or NaOH as MnO2 and Mn(OH)2, respectively, followed by Ni precipitation with dimethylglyoxime (DMG). The aqueous solution was further treated with 1 M NaOH solution for selective precipitation of Co as Co(OH)2, followed by Li precipitation as Li2CO3 by the addition of Na2CO3 solution. The purity of the recovered powder of Li, Mn, Co, and Ni was 96.97, 98.23, 96.94, and 97.43 wt.%, respectively. Gratz et al. [147] was able to co-precipitate the targeted NMC chemistry (Ni0.33Mn0.33Co0.33(OH)2) using NaOH with 90% metal recovery, which was further resynthesized as NMC cathode. Similar studies were done by He et al. [148] to recover spherical LiNi1/3Co1/3Mn1/3O2 cathode particles from spent LIBs with a carbonate co-precipitation method. Chemical precipitation is widely used in the industry due to its simple operations along with a low operational cost. However, a high purity product recovery with chemical precipitation is a challenge. Some relevant works from the literature for the value recovery from LIBs by chemical precipitation have been listed in Table 5 which shows the type of batteries recycled, operating conditions and the products obtained.
Closed Loop Hydrometallurgical Processes
Closing the loop is defined as recovering values from spent LIBs in a form that can be used in resynthesizing cathode active materials for new LIBs or other alternative materials. Thus, closing the loop will contribute to circular and sustainable processing of EV LIBs. Table 6 lists commercial and upcoming recycling processes for EV LIBs classified on the merits of closed-loop recycling, their recycling strategies, end products and capacities. The emerging technologies primarily employ hydrometallurgical unit operations and focus has been on recovering the values as cathode active material. After a detailed study of various established as well as emerging hydrometallurgy-based recycling processes coupled with our experience in LIB recycling, we summarize a general recovery scheme shown in Fig. 10 that reflects the growing need to consider environment, society and economics aspects of recycling choices. The process begins with battery discharging with NaCl [45, 48] solution to prevent electrocuting and short-circuiting (health hazards) followed by electrolyte recovery which can otherwise release toxic effluents (e.g. HF) (environmental concerns) and thermal treatment to remove binder [52, 53]. The thermal treatment is preferred over the usual solvent treatment (e.g. NMP) as they are toxic. The next step is magnetic separation or air classification to recover copper and aluminium. The anode and cathode material are separated by dense media separation processes or froth floatation process [53, 59, 60]. The cathode rich and anode rich streams are treated separately to recover high purity products. Cathode rich material is leached with inorganic acid, and metals values are recovered with chemical co-precipitation/solvent extraction. Although organic acids are more environmentally friendly, but they are expensive (economics concerns) and should be recycled to make the process sustainable. After leaching, NMC salts are recovered with chemical co-precipitation which has a distinct advantage to recover Ni, Mn, and Co together as they precipitate in the same pH range (9 to 11) [143, 147] as a precursor to the CAM in the subsequent stages, whereby closing the loop. Solvent extraction can be employed if one wishes to recover individual values separately with high purity but comes with additional cost, complexity and environmental concerns (economics & environment concerns) [136]. Finally, lithium is concentrated using evaporation followed by chemical precipitation. One way to avoid energy intensive evaporation is to up-concentrate the solution using solvent extraction or direct lithium extraction techniques, which is gaining wide popularity in the field [153, 154]. The co-precipitated NMC salt along with recovered Li salt is sintered to form cathode active material [147, 148]. Anode rich stream which is essentially graphite slurry with soluble Li leached (like water leaching [77]) during the froth flotation process is filtered and the solid fraction is treated to remanufacture anode material for new LIBs thereby closing the loop [53, 59, 60]. The liquid stream i.e., Li rich stream is processed along with the rest of the lithium in the lithium recovery process to recover lithium salt.
Direct Recycling
Direct recycling, which is a relatively new recycling technology regenerates the CAM from LIBs while maintaining its original structural integrity [155]. This method is more efficient than other pyro-hydrometallurgical methods because it recovers the functional cathode particle without decomposition into substituent elements. This also allows metal-oxide cathode materials to be reincorporated into a new cathode electrode with minimal changes to the crystal morphology of the active material [155]. Although this seems promising, it warrants rigorous sorting along with the control of impurities like Al and Cu [11]. After dismantling, binder is separated by dissolution in NMP or by pyrolysis at higher temperature (around 500 °C) followed by regeneration via solid state synthesis with addition of fresh Li2CO3 or by hydrothermal process with a solution containing Li2SO4/LiOH before annealing [14, 155,156,157]. Direct recycling is ideal for chemistries with low cobalt content. LFP chemistry is well suited for recycling through direct recycling. Li et al. [158]. in this work after dismantling and separation of spent LFP type spent LIBs (from EVs) regenerated cathode material using solid state synthesis at 650 °C and showed promising discharge capacity after 100 cycles. In another study by Chen et al. [159] for LCO chemistry, LCO cathode was resynthesized by thermal treatment at 850 °C with Li2CO3. The study also stated the negative impact of aluminium and copper impurities on electrochemical properties of resynthesized cathode. Shi et al. studied direct recycling of NMC 111 and NMC 532 chemistry spent LIBs and employed a hydrothermal method (2200C & 4 h) followed by an annealing step (8500C & 4 h in O2) [160]. The resynthesized NMC 111 and NMC 532 exhibited high capacity, good cycling stability, and high-rate performance [160]. In summary, direct recycling can be promising in recycling cathode materials which can be further used in the production of new LFP and LMO batteries [161]. But with the evolving battery technology and new cathode chemistries in the market, the feedstock for recycling process will be very diverse in the future and direct recycling technologies might struggle to achieve desired quality product.
Closing the Loop of LIB Value Chain
The unprecedented growth of LIBs around the world would put tremendous pressure on the reserves of Li, Co, Ni, Mn etc. which could result in new resource challenges and supply chain risks. It has been found that Li, Ni, Co, Mn oxide dominated battery scenario demand is estimated to increase by factors of 18–20 for Li, 17–19 for Co, 28–31 for Ni, and 15–20 for most other materials from 2020 to 2050 [162]. Li doesn’t occur in nature because of its reactivity and is mainly recovered from a salt solution like brines. Due to the expensive production cost and time consuming process, seawater Li extraction may not be central in future, unless new and innovative extraction methods are implemented. Co is mined primarily as a by-product of Ni and Cu and its market is highly centralized and 51% of reserves are in the Democratic Republic of Congo where mining comes with socio-economic exploitation [163, 164]. Moreover, the current reserves of these raw materials are not uniformly distributed across the world. For example, in 2019, most of the Li was extracted from Australia (55%) and Chile (24%); cobalt (70%) originated in the Democratic Republic of Congo; Ni (53%) originated from Indonesia, the Philippines, Russia, and New Caledonia; and graphite (60%) came from China [165]. In the wake of issues such as wars, pandemics, and sanctions, there might be an increase in import costs or limit/ban on raw materials. The governments across the world also recognize resource security from geographical and geopolitical factors and elements associated with LIBs such as Li and Co have been designated as strategic elements and critical materials by the EU. The conventional method of disposal of spent LIBs is through the landfill method which can cause groundwater or soil contamination as there is a risk of battery material leakage. Similarly, there is a risk of the formation of toxic compounds when the electrolyte from cracked batteries encounters the atmosphere or water. Battery recycling can address both challenges of shortage of raw material, as well as safe treatment of spent LIBs and eliminates the landfill requirement. A closed-loop LIBs recycling can lessen the stress on the finite raw material resources and environmental cost associated with the mining of virgin resources. The more critical contribution of recycling is towards the circular economy of EV LIBs which is vital for a robust raw material value chain. Hence there is a need to consider spent LIBs as raw material to tackle humungous future EV demand. In the Table 6, we have listed some large scale and pilot processes which have established closed loop recycling or have high potentials for doing so.
Conclusions
The governments around the world are pushing for transport electrification to reduce their carbon footprint and thus giving numerous incentives to promote EVs in the market. This has exponentially increased the demand of the metals contained in LIBs. In order to meet this demand, recycling of spent LIBs should be considered in addition to virgin metal mining. It is reflected from the critical review of the literature that hydrometallurgical recycling process has the potential to handle the recycling volumes and evolving LIB cathode chemistries. In this review we have critically evaluated the major steps in a typical hydrometallurgical based recycling process such as pretreatment, leaching, solvent extraction and chemical precipitation. Based on which we have recommended a process flowsheet which has the potential to recover all the components from a spent EV battery pack essentially closing the loop. In summary, mechanical pre-treatment is proposed since it is automated and suitable for commercial scale. Literature studies show H2SO4 and along with reducing agent H2O2 as the most favourable leaching solution. Chemical precipitation seems to be the more preferred extraction process as recovered values can be used to resynthesize the cathode active material to close the loop. There is a global push for EVs, but to make this transition successful, a robust raw material supply is imperative which makes recycling critical in terms of sustainability and circular economy for EV LIBs.
Future Outlooks
The future market looks promising for the EV industry with several countries encouraging their citizens to buy EVs through various incentives like tax benefits, government subsidies and special preferences on roads and parking lots [166]. There are ambitious plans by several countries to ban the sale of combustion engine vehicles within the next decade [167]. Many countries across the globe have already enacted regulations to push EV battery recycling e.g., New EU regulatory framework for batteries 2021, and also China [18, 168]. There needs to be further development in policy making coupled with greater focus on establishing and successfully expanding a robust recycling ecosystem.
The new EU regulatory framework for batteries 2021 has proposed extended producer responsibility (EPR) principle under which producer becomes responsible for waste management of batteries which they place in the market. The directive has given collection target of 65% by end of 2025 and 70% by the end of 2030 [168]. Certain technological areas require further thrust for successful implementation of the above ecosystem. There is a strong need to streamline spent LIBs collection from the end-user to the battery recycler which will help in sorting prior to dismantling while simultaneously preventing landfills. Although discharging is necessary to prevent the danger of electrocution, short-circuiting, and self-ignition, current discharging processes like dipping in brine solution or external discharger aren’t fit for expected commercial volumes. Hence there is a need to develop a safe and commercially viable spent LIBs discharging technology.
The present-day manual or mechanical dismantling needs to be replaced by automated dismantling technologies which shall be the pathway to safe handling of spent batteries. Most of the present commercial LIBs recycling technologies focus on Co and Ni recovery which dictates recycling feasibility, however, the newer cell chemistries will contain less of these materials, whereby requiring innovation in recycling methods. The upcoming processes such as ONTO Process, Battery Resources Process & LithoRec Process are focusing on recovering cathode active material from LIBs waste—such and other upcoming processes may hold a key to improved circularity. There is little work on graphite and electrolyte recovery, primarily due to low profitability. In this field, there needs to be focussed research on resynthesis of anode materials and reclaim of electrolyte, binder, etc. Although this industry has seen state-of-the-art hydrometallurgical processes in operation, there is little focus on acid recycling for minimization of liquid effluent, an area that requires attention in the future. Furthermore, direct Recycling, an up-and-coming technology, should be investigated further for handling different battery chemistries and subsequent upscaling or commercialization.
Our optimism is that the future recycling processes for LIBs will be closed loop and combine both hydrometallurgy and pyrometallurgy. The metal recovery will be in the form of cathode active material ready to be remanufactured as a cathode and the graphite will be resynthesized as well. However, here, it may be emphasized that with a growing diversity in new battery chemistries, recycling technologies need to evolve rapidly. For a co-ordinated ecosystem, there needs to be closer collaboration between battery designers, policy makers and recyclers to incorporate design for recycling.
References
International Energy Agency (2021) Global EV outlook 2021: Accelerating ambitions despite the pandemic
Goldman Sachs (2023) Electric vehicles are forecast to be half of global car sales by 2035. https://www.goldmansachs.com/intelligence/pages/electric-vehicles-are-forecast-to-be-half-of-global-car-sales-by-2035.html. Accessed April 2023
Kapustin NO, Grushevenko DA (2020) Long-term electric vehicles outlook and their potential impact on electric grid. Energy Policy 137:111103
Norwegian Electric Vehicle Association (2022) Norwegian EV market. https://elbil.no/english/norwegian-ev-market/. Accessed December 2022.
Wang N, Tang L, Pan H (2019) A global comparison and assessment of incentive policy on electric vehicle promotion. Sustain Cities Soc 44:597–603
Sumangil AYM (2021) Top electric vehicle markets dominate lithium-ion battery capacity growth. S&P Global Market Intelligence, New York
Holland DA (2021) Li-ion battery recycling market 2022–2042
Meshram P, Pandey BD, Mankhand TR (2015) Recovery of valuable metals from cathodic active material of spent lithium ion batteries: leaching and kinetic aspects. Waste Manag 45:306–313
Barik SP, Prabaharan G, Kumar B (2016) An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag 51:222–226
Pinegar H, Smith YR (2019) Recycling of end-of-life lithium ion batteries, Part I: commercial processes. J Sustain Metall 5(3):402–416
Chagnes A, Pospiech B (2013) A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J Chem Technol Biotechnol 88(7):1191–1199
Chen X et al (2015) Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag 38:349–356
Chen M et al (2019) Recycling end-of-life electric vehicle lithium-ion batteries. Joule 3(11):2622–2646
Harper G et al (2019) Recycling lithium-ion batteries from electric vehicles. Nature 575(7781):75–86
Li L et al (2018) The recycling of spent lithium-ion batteries: a review of current processes and technologies. Electrochem Energy Rev 1(4):461–482
Pinegar H, Smith YR (2020) Recycling of end-of-life lithium-ion batteries, Part II: laboratory-scale research developments in mechanical, thermal, and leaching treatments. J Sustain Metall 6(1):142–160
Yoshio M, Brodd RJ, Kozawa A (2009) Lithium-ion batteries, vol 1. Springer, New York
Zhang X et al (2018) Toward sustainable and systematic recycling of spent rechargeable batteries. Chem Soc Rev 47(19):7239–7302
Mishra A et al (2018) Electrode materials for lithium-ion batteries. Mater Sci Energy Technol 1(2):182–187. https://doi.org/10.1016/j.mset.2018.08.001
Oswal M, Paul J, Zhao R (2010) A comparative study of lithium-ion batteries. University of Southern California
Fujita T et al (2021) Reduction, reuse and recycle of spent Li-ion batteries for automobiles: A review. Int J Miner Metall 28(2):179–192
Fraser J, Anderson J, Lazuen J, Lu Y, Heathman O, Brewster N, Bedder J and Masson O (2021) Study on future demand and supply security of nickel for electric vehicle batteries. Publications Office of the European Union, Luxembourg
Brückner L, Frank J and Elwert TJM ( 2020) Industrial recycling of lithium-ion batteries—A critical review of metallurgical process routes. Metals 10(8):1107
Armand M, et al (2020) Lithium-ion batteries–Current state of the art and anticipated developments. J Power Sources 479:228708
Greenwood M, Wentker M, Leker J (2021) A bottom-up performance and cost assessment of lithium-ion battery pouch cells utilizing nickel-rich cathode active materials and silicon-graphite composite anodes. J Power Sources Adv 9:100055
Casimir A et al (2016) Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 27:359–376. https://doi.org/10.1016/j.mattod.2014.10.040
Nitta N et al (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Makuza B et al (2021) Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J Power Sources 491:229622. https://doi.org/10.1016/j.jpowsour.2021.229622
Zhu P et al (2021) A review of current collectors for lithium-ion batteries. J Power Sources 485:229321
Li Q et al (2016) Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ 1(1):18–42
Jang J et al (2020) A review of functional separators for lithium metal battery applications. Materials 13(20):4625
Franco Gonzalez A, Yang N-H and Liu R-S (2017) Silicon Anode Design for Lithium-Ion Batteries: Progress and Perspectives. J Phys Chem C 121(50):27775–27787.
Xu W et al (2014) Lithium metal anodes for rechargeable batteries. Energy Environ Sci 7(2):513–537
Chauque S et al (2017) Lithium titanate as anode material for lithium ion batteries: Synthesis, post-treatment and its electrochemical response. J Electroanal Chem 799:142–155
Gunawan I, Sugeng B (2017) Synthesis and characterization of PVA blended LiClO4 as electrolyte material for battery Li-ion. in IOP Conference Series: Materials Science and Engineering. IOP Publishing
Elia GA et al (2014) Role of the Lithium Salt in the Performance of Lithium-Oxygen Batteries: A Comparative Study. ChemElectroChem 1(1):47–50
Zheng F et al (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389:198–213
Lizundia E, Kundu D (2021) Advances in Natural Biopolymer-Based Electrolytes and Separators for Battery Applications. Adv Func Mater 31(3):2005646
Wikner E, (2017) Lithium ion battery aging: battery lifetime testing and physics-based modeling for electric vehicle applications. Chalmers Tekniska Hogskola (Sweden).
Greim P, Solomon A, Breyer CJNc (2020) Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat Commun 11(1):1–11
Lih W-C et al (2012) Second use of retired lithium-ion battery packs from electric vehicles: technological challenges, cost analysis and optimal business model. in 2012 International Symposium on Computer, Consumer and Control. IEEE, 2012
Sonoc A, Jeswiet J, Soo VK (2015) Opportunities to Improve Recycling of Automotive Lithium Ion Batteries. Procedia CIRP 29:752–757
Xie Y et al (2017) Progress in traction battery recycling industry for electric vehicles. In: Proc. 5th Int Conf. Mechatronics, Mater. Chem. Comput. Eng.(ICMMCCE)
Sommerville R et al (2020) A review of physical processes used in the safe recycling of lithium ion batteries. Sustain Mater Technol 25:e00197
Yao LP et al (2020) An environmentally friendly discharge technology to pretreat spent lithium-ion batteries. J Clean Prod 245:118820
Lu M et al (2013) The re-synthesis of LiCoO2 from spent lithium ion batteries separated by vacuum-assisted heat-treating method. Int J Electrochem Sci 8(6):8201–8209
Ojanen S et al (2018) Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling. Waste Manag 76:242–249
He L-P et al (2017) Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustainable Chem Eng 5(1):714–721
Li L et al (2012) Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J Power Sources 218:21–27
Li L et al (2014) Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J Power Sources 262:380–385
Pant D, Dolker TJWM (2017) Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) based Lithium ion batteries. Waste Manag 60:689–695
Xiao J, Li J Xu ZJJohm (2017) Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J Hazard Mater 338:124–131.
Wang F et al (2018) Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J Clean Prod 185:646–652
Zhong X et al (2020) Pretreatment for the recovery of spent lithium ion batteries: theoretical and practical aspects. J Clean Prod 263:121439
Martínez OV et al (2019) Statistical entropy analysis as tool for circular economy: Proof of concept by optimizing a lithium-ion battery waste sieving system. J Clean Prod 212:1568–1579
Marinos D, Mishra BJJoSM (2015) An Approach to processing of lithium-ion batteries for the zero-waste recovery of materials. J Sustain Metall 1(4):263–274
Zhang T et al (2014) Surface analysis of cobalt-enriched crushed products of spent lithium-ion batteries by X-ray photoelectron spectroscopy. Sep Purif Technol 138:21–27
Vanderbruggen, A., et al., (2021) A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling.. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021: p. 127111.
He Y et al (2017) Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J Clean Prod 143:319–325
Shin H et al (2020) Electrochemical performance of recycled cathode active materials using froth flotation-based separation process. J Electrochem Soc 167(2):020504
Wang M-M, Zhang C-C, Zhang F-SJWM (2017) Recycling of spent lithium-ion battery with polyvinyl chloride by mechanochemical process. Waste Manag 67:232–239
Nayaka G et al (2016) Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 161:54–57
Xin Y et al (2016) Bioleaching of valuable metals Li Co. Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. J Clean Prod 116:249–258
Li L et al (2013) Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J Power Sources 233:180–189
Tan WJ et al (2021) A hybrid disassembly framework for disassembly of electric vehicle batteries. Int J Energy Res 45(5):8073–8082
Wegener K et al (2015) Robot Assisted Disassembly for the Recycling of Electric Vehicle Batteries. Procedia CIRP 29:716–721
Wegener K et al (2014) Disassembly of Electric Vehicle Batteries Using the Example of the Audi Q5 Hybrid System. Procedia CIRP 23:155–160
Zhou L et al (2021) Battery pack recycling challenges for the year 2030: Recommended solutions based on intelligent robotics for safe and efficient disassembly, residual energy detection, and secondary utilization. Energy Storage 3(3):e190
Lombardo G et al (2021) Comparison of the effects of incineration, vacuum pyrolysis and dynamic pyrolysis on the composition of NMC-lithium battery cathode-material production scraps and separation of the current collector. Resour Conserv Recycl 164:105142
Zheng R et al (2016) Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Advances 6(49):43613–43625
Zhang X et al (2016) Sustainable recycling and regeneration of cathode scraps from industrial production of lithium-ion batteries. ACS Sustainable Chem Eng 4(12):7041–7049
Bi H et al (2021) Low-temperature thermal pretreatment process for recycling inner core of spent lithium iron phosphate batteries. Waste Manag Res 39(1):146–155
Sun L, Qiu K (2011) Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J Hazard Mater 194:378–384
Zhang G et al (2018) Pyrolysis-ultrasonic-assisted flotation technology for recovering graphite and LiCoO2 from spent lithium-ion batteries. ACS Sustainable Chem Eng 6(8):10896–10904
Vieceli N et al (2021) Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag 125:192–203
Träger T, Friedrich B, Weyhe RJCit (2015) Recovery Concept of Value Metals from Automotive Lithium‐Ion Batteries 2015. Chem Ing Tech 87(11):1550–1557
Higuchi A et al (2016) Selective recovery of lithium from cathode materials of spent lithium ion battery. JOM 68(10):2624–2631
Hu J et al (2017) A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J Power Sources 351:192–199
Zhang G et al (2021) Recent advances in pretreating technology for recycling valuable metals from spent lithium-ion batteries. J Hazard Mater 406:124332
Kim S et al (2021) A comprehensive review on the pretreatment process in lithium-ion battery recycling. J Clean Prod 294:126329
Yu D et al (2021) Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. Miner Eng 173:107218
Chen X et al (2015) Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag 38:349–356
Fajaryanto R, Nurqomariah A (2018) Acid leaching and kinetics study of cobalt recovery from spent lithium-ion batteries with nitric acid. In: E3S Web of Conferences. EDP Sciences
Porvali A et al (2019) Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste. Resour Conserv Recycl 142:257–266
Yu M et al (2019) A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep Purif Technol 215:398–402
Zeng X, Li J, Shen B (2015) Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid J Hazard Mater 295:112–118
Sun C et al (2018) Sustainable recovery of valuable metals from spent lithium-ion batteries using DL-malic acid: Leaching and kinetics aspect. Waste Manag Res 36(2):113–120
Li L et al (2017) Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustainable Chem Eng 5(6):5224–5233
Qi Y et al (2020) A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J Clean Prod 251:119665
Srichandan H et al (2019) Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 189:105122
Lee CK, Rhee K-IJH (2003) Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 68(1–3):5–10
Meshram P, Pandey BD, Mankhand TR, (2015) Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem Eng J 281:418–427
Pinna EG et al (2017) Cathodes of spent Li-ion batteries: Dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors. Hydrometallurgy 167:66–71
Golmohammadzadeh R, Rashchi F, Vahidi EJWM (2017) Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag 64:244–254
Shin SM et al (2005) Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 79(3):172–181
Zheng R et al (2017) A closed-loop process for recycling LiNixCoyMn(1–x−y)O2 from mixed cathode materials of lithium-ion batteries. Green Energy & Environment 2(1):42–50
Zhao J et al (2020) Hydrometallurgical recovery of spent cobalt-based lithium-ion battery cathodes using ethanol as the reducing agent. Environ Res 181:108803
Chen X et al (2018) Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries. Waste Manage 75:459–468
Zhang P et al (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47(2–3):259–271
Wang R-C, Lin Y-C, Wu S-H (2009) A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 99(3):194–201
Castillo S et al (2002) Advances in the recovering of spent lithium battery compounds. J Power Sources 112(1):247–254
Lee CK, Rhee K-I (2002) Preparation of LiCoO2 from spent lithium-ion batteries. J Power Sources 109(1):17–21
Theron MM, Lues JR (2010) Organic acids and food preservation. CRC press
Mulligan CN, Kamali M, Gibbs BF (2004) Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J Hazard Mater 110(1–3):77–84
Fan B et al (2016) A sustainable process for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag Res 34(5):474–481
Wang S et al (2020) Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: effect of reductants and ammonium salts. Waste Manag 102:122–130.
Meng K et al (2019) Comparison of the Ammoniacal Leaching Behavior of Layered LiNi x Co y Mn1–x–y O2 (x= 1/3, 0.5, 0.8) Cathode Materials. ACS Sustainable Chem Eng 7(8):7750–7759
Zheng X et al (2017) Spent lithium-ion battery recycling–Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag 60:680–688
Wang C et al (2020) Recycling of spent lithium-ion batteries: Selective ammonia leaching of valuable metals and simultaneous synthesis of high-purity manganese carbonate. Waste Manag 114:253–262
Ma Y et al (2021) A promising selective recovery process of valuable metals from spent lithium ion batteries via reduction roasting and ammonia leaching. J Hazard Mater 402:123491
Or T et al (2020) Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2(1):6–43
Moazzam P et al (2021) Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere 277:130196
Xin B et al (2009) Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. Biores Technol 100(24):6163–6169
Heydarian A et al (2018) Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J Power Sources 378:19–30
Jegan Roy J et al (2021) Bioleaching as an Eco-Friendly Approach for Metal Recovery from Spent NMC-Based Lithium-Ion Batteries at a High Pulp Density. ACS Sustainable Chem Eng 9(8):3060–3069
Sattar R et al (2019) Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep Purif Technol 209:725–733
Chen W-S, Ho H-J (2018) Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals 8(5):321
Wang J et al (2012) Leaching Study of Spent Li-ion Batteries. Procedia Environ Sci 16:443–450
Lee CK, Rhee K-I (2003) Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 68(1):5–10
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 32(8):1575–1582
Nayaka GP et al (2016) Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag 51:234–238
Gao R et al (2020) Recycling of LiNi0. 5Co0. 2Mn0. 3O2 Material from Spent Lithium‐ion Batteries Using Mixed Organic Acid Leaching and Sol‐gel Method. ChemistrySelect 5(21):6482–6490
Roy JJ, Madhavi S, Cao B (2021) Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. J Clean Product 280:124242
Joo S-H, et al (2015) Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosene. Hydrometallurgy 156:136–141
Torkaman R et al (2017) Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process. Sep Purif Technol 186:318–325
Kang J et al (2010) Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 100(3–4):168–171
Wesselborg T, Virolainen S, Sainio TJH (2021) Recovery of lithium from leach solutions of battery waste using direct solvent extraction with TBP and FeCl3. Hydrometallurgy 202:105593
Wang F et al (2016) Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid–liquid separation and solvent extraction. RSC Advances 6(88):85303–85311
Swain B et al (2007) Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J Power Sources 167(2):536–544
Nan J, Han D, Zuo X (2005) Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J Power Sources 152:278–284
Joo S-H et al (2016) Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy 159:65–74
Chen X, Zhou T, (2014) Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag Res 32(11):1083–1093
Tang W et al (2014) Recovery of Ti and Li from spent lithium titanate cathodes by a hydrometallurgical process. Hydrometallurgy 147:210–216
Joo S-H, et al (2017) Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 Petroleum Liquid Catalyst from the Cathode Material of Spent Lithium Ion Batteries by a Hydrometallurgical Route. Metals 7(10):439
Nayl A., Hamed MM, Rizk SE (2015) Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries. J Taiwan Inst Chem 55:19–125
Nan J et al (2006) Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 84(1–2):75–80
Chen X et al (2015) Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep Purif Technol 144:197–205
Swain B et al (2008) Development of process flow sheet for recovery of high pure cobalt from sulfate leach liquor of LIB industry waste: A mathematical model correlation to predict optimum operational conditions. Sep Purif Technol 63(2):360–369
Pagnanelli F et al (2016) Cobalt products from real waste fractions of end of life lithium ion batteries. Waste Manage 51:214–221
Granata G et al (2012) Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: Accomplishment of European Guidelines by optimizing mechanical pre-treatment and solvent extraction operations. J Power Sources 212:205–211
Fernandes A, Afonso JC, Bourdot Dutra AJ, (2012) Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. J Power Sources 220:286–291
Ojanen S et al (2018) Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling. Waste Manage 76:242–249
Chen M et al (2019) Closed Loop Recycling of Electric Vehicle Batteries to Enable Ultra-high Quality Cathode Powder. Sci Rep 9(1):1654
Zhu S-G et al (2012) Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans Nonferrous Met Soc China 22(9):2274–2281
Meshram P, Pandey BD, Mankhand TR (2015) Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem Eng J 281:418–427
Pegoretti VCB et al (2017) Thermal synthesis, characterization and electrochemical study of high-temperature (HT) LiCoO2 obtained from Co(OH)2 recycled of spent lithium ion batteries. Mater Res Bull 86:5–9
Gratz E et al (2014) A closed loop process for recycling spent lithium ion batteries. J Power Sources 262:255–262
He L-P, Sun S-Y, Yu J-G (2018) Performance of LiNi1/3Co1/3Mn1/3O2 prepared from spent lithium-ion batteries by a carbonate co-precipitation method. Ceram Int 44(1):351–357
Li J et al (2009) A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 77(8):1132–1136
Wang M-M, Zhang C-C, Zhang F-S (2016) An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach. Waste Manage 51:239–244
Zheng R et al (2016) Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv 6(49):43613–43625
Cai G et al (2014) Process Development for the Recycle of Spent Lithium Ion Batteries by Chemical Precipitation. Ind Eng Chem Res 53(47):18245–18259
Zhang L et al (2020) Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction. J Hazard Mater 398:122840
Stringfellow WT, Dobson PF (2021) Technology for the Recovery of Lithium from Geothermal Brines. Energies 14(20):6805
Sloop S et al (2020) A direct recycling case study from a lithium-ion battery recall. Sustain Mater Technol 25:e00152
Yang T et al (2020) An Effective Relithiation Process for Recycling Lithium-Ion Battery Cathode Materials. Advanced Sustainable Systems 4(1):1900088
Gao H et al (2020) Efficient Direct Recycling of Degraded LiMn2O4 Cathodes by One-Step Hydrothermal Relithiation. ACS Appl Mater Interfaces 12(46):51546–51554
Li X et al (2017) Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J Power Sources 345:78–84
Chen S et al (2016) Renovation of LiCoO2 with outstanding cycling stability by thermal treatment with Li2CO3 from spent Li-ion batteries. J Energy Storage 8:262–273
Shi Y et al (2018) Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett 3(7):1683–1692
Dunn JB et al (2012) Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ Sci Technol 46(22):12704–12710
Xu C et al (2020) Future material demand for automotive lithium-based batteries. Commun Mater 1(1):1–10
Baars J et al (2021) Circular economy strategies for electric vehicle batteries reduce reliance on raw materials. Nat Sustain 4(1):71–79
Kushnir D, Sandén BA, (2012) The time dimension and lithium resource constraints for electric vehicles. Resour Policy 37(1):93–103
Ali H, Khan HA, Pecht MG, (2021) Circular economy of Li Batteries: Technologies and trends.J Energy Storage 40:102690
Arambarri J et al (2019) Lithium ion car batteries: present analysis and future predictions. Environ Eng Res 24(4):699–710
He X et al (2019) Research on the energy efficiency of energy regeneration systems for a battery-powered hydrostatic vehicle. Energy 178:400–418
Halleux V (2021) New EU regulatory framework for batteries: setting sustainability requirements
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleem, U., Joshi, B. & Bandyopadhyay, S. Hydrometallurgical Routes to Close the Loop of Electric Vehicle (EV) Lithium-Ion Batteries (LIBs) Value Chain: A Review. J. Sustain. Metall. 9, 950–971 (2023). https://doi.org/10.1007/s40831-023-00718-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00718-w