Abstract
Background
Without appropriate prophylaxis, the rate of vertical transmission of hepatitis B virus (HBV) can be as high as 95%. Alberta’s provincial prenatal program screens all pregnant women for HBV, and provides prophylaxis to infants born to HBV-infected women. Canadian data on the outcomes of such programs are limited.
Methods
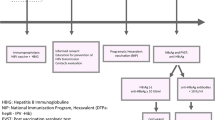
We conducted a retrospective review of data from pregnant Albertan women who were Hepatitis B Surface Antigen (HBsAg) positive from 1997–2004. We describe the frequency of hepatitis B immunoglobulin (HBIG) and vaccine administration, follow-up serology and pregnancy outcomes.
Results
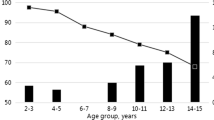
In total, 1,485 HBsAg-positive pregnant women were identified; an average of 186 women annually (range: 125–216). Of the 980 infants eligible to have completed prophylaxis and serological follow-up, 82.0% were appropriately immunized and serologically tested, 11.3% had complete immunization but no serology testing and 6.6% were incompletely immunized. Of infants with complete immunization and follow-up, 3.7% failed to mount an immune response and 2.1% were infected.
Conclusion
A high proportion of infants born to carrier mothers are receiving appropriate post-natal prophylaxis in Alberta. Future research should examine maternal factors that may increase the vertical transmission of HBV.
Résumé
Contexte
Sans prophylaxie appropriée, le taux de transmission verticale du virus de l’hépatite B (VHB) peut atteindre 95 %. Dans le cadre du programme prénatal provincial de l’Alberta, toutes les femmes enceintes sont testées pour le VHB, et la prophylaxie est offerte aux nourrissons de femmes infectées par le virus. Les données canadiennes sur les résultats de tels programmes sont limitées.
Méthode
Nous avons mené une étude rétrospective auprès des femmes enceintes de l’Alberta qui étaient porteuses de l’antigène de surface de l’hépatite B (AgHBs) entre 1997 et 2004. Nous avons décrit la fréquence de l’administration de l’immunoglobuline anti-hépatite B (HBIg) et du vaccin contre le VHB, les résultats des tests sérologiques et les résultats de grossesse.
Résultats
Sur l’ensemble des femmes enceintes testées, 1 485 porteuses de l’AgHBS ont été identifiées, soit en moyenne 186 femmes par année (125 à 216). Des 980 nourrissons admissibles à la prophylaxie complète et au suivi sérologique, 82 % avaient reçu tous les vaccins ainsi que les tests sérologiques, 11,3 % avaient reçu les vaccins mais aucun test sérologique, et 6,6 % n’avaient pas été complètement immunisés. Sur les nourrissons immunisés et ayant fait l’objet d’un suivi sérologique, 3,7 % n’avaient pas manifesté de réponse immunitaire, et 2,1 % étaient infectés.
Conclusion
En Alberta, une proportion élevée de nourrissons de mères infectées par le VHB reçoit la prophylaxie postnatale appropriée. D’autres études sont nécessaires pour déterminer les facteurs maternels qui augmentent le risque de transmission du VHB de la mère à l’enfant.
Similar content being viewed by others
References
NACI. Canadian Immunization Guide, 6th edition. Canadian Medical Institution, 2002.
Tran TT, Martin P. Hepatitis B: Epidemiology and natural history. Clin Liver Dis 2004;8(2):255–66.
Stevens CE, Taylor PE, Tong MJ, Toy PT, Vyas GN, Nair PV, et al. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA 1987;257(19):2612–16.
Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B imuneglobulin and hepatitis B vaccine. Lancet 1983;2(8359):1099–102.
Alberta Bloodborne Pathogens Surveillance Working Group, Alberta Bloodborne Pathogens Surveillance Report. Alberta Health and Wellness: Edmonton, AB, 2003.
Minuk G, Uhanova J. Chronic hepatitis B in Canada. Can J Infect Dis 2001;12(6):351–56.
Zhang J, Zou S, Giulivi A. Hepatitis B in Canada. CCDR 2001;27S3:10–12.
Henning KJ, Pollack DM, Friedman, SM. A neonatal hepatitis B surveillance and vaccination program: New York City, 1987 to 1988. Am J Public Health 1992;82(6):885–88.
Kohn MA, Farley TA, Scott C. The need for more aggressive follow-up of children born to hepatitis B surface antigen-positive mothers: Lessons from the Louisiana Perinatal Hepatitis B Immunization Program. Pediatr Infect Dis J 1996;15(6):535–40.
Chernesky MA, Blajchman MA, Castriciano S, Basbaum J, Spiak C, Mahoney, JB. Analysis of a pregnancy-screening and neonatal-immunization program for hepatitis B in Hamilton, Ontario, Canada, 1977–1988. J Med Virol 1991;35(1):50–54.
Okun NB, Larke RP, Waters JR, Joffres, MR. Success of a program of routine prenatal screening for hepatitis B surface antigen: The first 2 years. CMAJ 1990;143(12):1317–21.
Wong S, Chan LY, Yu V, Ho L. Hepatitis B carrier and perinatal outcome in singleton pregnancy. Am J Perinatol 1999;16(9):485–88.
del Canho R, Grosheide PM, Mazel JA, Heijtink RA, Hop WC, Gerards LJ, et al., Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982–1992: Protective efficacy and long-term immunogenicity. Vaccine 1997;15(15):1624–30.
Mandelbrot L, Newell M-L, Vertical transmission of hepatitis viruses. In: Newell M-L, McIntyre J (Eds.), Congenital and Perinatal Infections: Prevention, Diagnosis and Treatment. Cambridge, UK: Cambridge University Press, 2000;164–204.
Lee SD, Lo KJ, Tsai YT, Wu, JC. Maternal hepatitis B virus DNA in mother-infant transmission. Lancet 1989;1(8640):719.
Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley, RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994;170(6):1418–23.
del Canho R, Grosheide PM, Schalm SW, de Vries RR, Heijtink, RA. Failure of neonatal hepatitis B vaccination: The role of HBV-DNA levels in hepatitis B carrier mothers and HLA antigens in neonates. J Hepatol 1994;20(4):483–86.
Corneil T, Gilbert M, Buxton J, Krajden M, McNabb G, McIntyre C. The Use of Routine HBeAg Testing in Pregnant Women in British Columbia to Identify Those Infants at Highest Risk for Vertical Transmission. Presented at the 6th Annual Canadian Immunization Conference. Montreal, Canada, 2004.
Reproductive Health Report Working Group, Alberta Reproductive Health: Pregnancies and Births 2004. Alberta Health and Wellness: Edmonton, AB.
van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen, HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003;10(4):294–97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plitt, S.S., Somily, A.M. & Singh, A.E. Outcomes from a Canadian Public Health Prenatal Screening Program for Hepatitis B. Can J Public Health 98, 194–197 (2007). https://doi.org/10.1007/BF03403711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03403711