Abstract
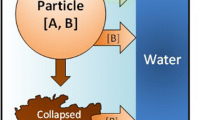
A kinetic model for the batch leaching of various metals using different lixiviants was developed. The overall material balances for the reactants and products at the solid/liquid interface were formulated for various types of heterogeneous reactions at the interface. The solutions to these mass balances are identified analytically, as well as numerically. Applications of these numerical solutions to various leaching systems using rotating discs or powders are discussed. The metal-dissolution systems addressed include cobalt, nickel and copper in acid, cyanide and ammoniacal solutions.
Similar content being viewed by others
Abbreviations
- a, b, c, d:
-
Stoichiometric coefficients for reaction (1)
- β:
-
ratio of a/c
- CAb:
-
Concentration of reactant A in the bulk solution
- CAs:
-
Interfase concentration of reactant A
- CBs:
-
Interfase concentration of reactant B
- CDs:
-
Concentration of product D at the interphase
- Cpb:
-
Concentration of product P in the bulk solution
- Cps:
-
Concentration of product P at the interphase
- C0ps:
-
Initial concentration of product P at the interphase
- C0Ab:
-
Initial concentration of product P in the bulk solution
- Di:
-
Diffusivity of i species
- d:
-
diameter of particulate
- Kmi, kmA:
-
Mass transfer coefficients of i and A, respectively
- km1, km2:
-
As given in the text
- kmr, kmp:
-
Mass transfer coefficients of reactants and products
- kf:
-
Forward rate constant
- kb:
-
Backward rate constant
- k1, k2:
-
As given in the text
- l, m, q, x:
-
Reaction orders of Eq. (1) with respect to individual species
- A, B:
-
Reactant species
- D, P:
-
Product species
- M, G:
-
Integral constants as defined in Eq. (4)
- M′:
-
Metal substrate, solid reactant
- n:
-
V/v
- R:
-
Percentage of km1 contributing to the overall rate
- S:
-
Surface area of solid metal; t Leaching time
- v:
-
Volume of the bulk solution
- vs:
-
Slip velocity
- δ:
-
Thickness of diffusion boundary layer
- δ′:
-
Thickness of interface
- θ:
-
The fraction of surface unavailable for the reaction; ν Kinematic viscosity of fluid
- ω:
-
Angular velocity of rotating disc.
References
Bhuntumkomol, K., Han, K.N., and Lawson, F., 1980, “Leaching behavior of metallic nickel in ammonia solutions,” Transactions of Institute Mining Metallurgy, Sec. C, C7-13.
Choi, Y., Lee, E.C., and Han, K.N., 1991, “The dissolution behavior of metals from Ag/Cu and Ag/Au alloys in acidic and cyanide solutions,” Met Transactions B, Vol. 22B, pp. 755–765.
Guan, Y., and Han, K.N., 1993, The dissolution behavior of gold and copper from gold and copper alloys, Miner. Metallurgy Proc. Vol. 10, pp. 66–74.
Habashi, F., 1967, “Kinetics and mechanism of gold and silver dissolution in cyanide solution,” Bulletin of Bureau of Mines and Geology, Vol. 59.
Han, K.N., and Lawson, F., 1974, “Leaching behavior of cobalt in acid solutions,” Journal of Less-common Metals, Vol. 38, pp. 19–29.
Han, K.N., and Vu, C., 1981, “A kinetic model for leaching of cobalt metal powder in NH3-H2O systems.” Hydrometallurgy, Vol. 6, pp. 227–238.
Hirato, T., et al., 1989, “The Leaching of sintered CuS disks with ferric chlorides,” Metallurgical Transactions B, 20B, pp. 485–491.
Ke, J.-J., Yue, L.-D., and Liu, W.-D., 1986, “Kinetics of dissolution of synthetic scheelite by an alkaline EDTA leach solution,” Hydrometallurgy, 16, pp. 325–334.
Kolodziej, B., 1988, “Digestion of silver in acidic ferric chloride and copper chloride solutions,” Hydrometallurgy, 20, pp. 219–233.
Lee, L.S.Y., and Lawson, F., 1989, “The leaching rate of tin metal in oxygenated sodium hydroxide solution,” Hydrometallurgy, 3, pp. 23–35.
Levich, V.G., 1962, Physicochemical Hydrodynamics, 2nd ed., Prentice-Hall, Englewood Cliffs, NJ, p. 700.
Meng, X., and Han, K.N., “A kinetic model for dissolution of metals — The role of heterogeneous reaction and mass transfer,” Minerals and Metallurgical Processing, Vol. 10, pp. 128–134.
Meng, X., and Han, K.N., 1993, “The dissolution behavior of gold in ammoniacal solutions,” Proceedings of the IV International Symposium on Hydrometallurgy, SME, pp. 205–221.
Meng, X., and Han, K.N., 1994, The dissolution behavior of cobalt in iodide/iodine solutions, Presented at SME Annual Meeting, Albuquerque, NM, Feb.
Meng, X., and Ke, J., 1987, “Study on leaching kinetics of Ag in ammoniacal solutions,” Acta Metallurqica Sinica (Chinese) Vol. 23, No. 2. pp. B68–75.
Morrison, R.M., 1989, “The dissolution of silver in ferric sulphate-sulphuric acid media,” Hydrometallurgy, Vol. 22, pp. 67–85.
Pesic, B., and Seal, T., 1990, “A rotating disk study of silver dissolution with thiourea in the presence of ferric sulfate,” Metallurgical Transactions B, Vol. 21B, pp. 419–427.
Qi, P.H., and Hiskey, J.B., “Dissolution kinetics of gold on iodide solutions,” Hydrometallurgy, Vol. 27, pp. 47–62.
Stephenson, R., 1971, Computer Simulation for Engineers, Harcourt, Brace, and Jovanovich.
Vu, C., and Han, K.N., 1977, “Leaching behavior of cobalt in ammonia solutions,” Transactions of the Institute of Mining and Metallurgy, Sec. C., Vol. 86, pp. C119–125.
Vu, C., and Han, K.N., 1979, “Effect of system geometry on the leaching behavior of cobalt metal: mass transfer controlling case,” Metallurgical Transactions B, Vol. 10B, pp. 57–62.
Author information
Authors and Affiliations
Additional information
SME Preprint 94–29, SME Annual Meeting, Feb. 14–17, 1994, Albuquerque, NM.
M&MP paper 94–618. Discussion of this peer-reviewed and approved paper is invited and must be submitted, in duplicate, prior to Aug. 31, 1995.
Rights and permissions
About this article
Cite this article
Meng, X., Sun, X. & Han, K.N. A dissolution kinetic model for metals in solutions. Mining, Metallurgy & Exploration 12, 97–102 (1995). https://doi.org/10.1007/BF03403085
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03403085