Abstract
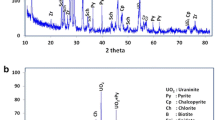
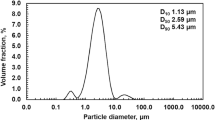
The leaching of the major mineral (potassium alunite) from alunite ores with a potassium hydroxide solution is studied. The process occurs with the retention of other minerals (dickite, hematite, quartz) in the leaching residues. The kinetics of leaching of potassium alunite from alunite raw materials is studied by the powder dissolution method in the temperature range of a potassium hydroxide solution 333–368 K at a KOH concentration of 52.96–243.8 g/L. The activation energy of the process is calculated using the experimental results obtained (Ea = 73.14 kJ/mol). The calculated energy indicates that the leaching of alunite from alunite ores is controlled by a surface chemical reaction, and the reaction order with respect to KOH is 1.02. The process under study is described by a first-order kinetic equation.






Similar content being viewed by others
REFERENCES
K. W. Loast, “Recovery of aluminium from alunite using acid leaching to purity the residue for Bayer leach,” VP Patent 4.031.182, 1977.
L. J. Froisland, M. L. Wouden, and D. D. Harbuck, “Acid sulfation of alunite,” in United States Bureau of Mines Report (1989), R19222.
W. Zhao, X. Yao, S. P. Zhong, Y. Y. Zhu, X. Yang, L. Yi, G. Li, J. Song, H. Yu, R. Ruan, and T. Qi, “Extraction of Al and K salts from associated alunite tailings by an acid calcination—water leaching method,” J. Clean. Prod. 107, 792–798 (2015).
M. Özdemir and H. Cetisli, “Extraction kinetics of alunite in sulfuric acid and hydrochloric acid,” Hydrometallurgy 76, 217–224 (2005).
G. Z. Nasyrov, E. I. Zemlyanskaya, and I. V. Ravndonikas, “Process for alunite treatment,” US Patent 4.117.077, 1978.
M. Ozacar and L. Sengil, “Optimum conditions for leaching calcined alunite ore in strong NaOH,” Can. Metall. 38 (4), 249–255 (1999).
M.-J. Luo, C.-L. Liu, J. Xue, P. Li, and J.-G. Yu, “Leaching kinetics and mechanism of alunite leaching from alunite tailings in highly concentrated KOH solution,” Hydrometallurgy 174, 10–20 (2017).
M. Mohammadi and M. M. Salarirad, “Kinetics of direct leaching of natural alunite in KOH,” Ind. Eng. Chem. Res. 52 (40), 1459–1465 (2013).
A. I. Zelikman, T. M. Vol’dman, and L. V. Vel’yavskaya, Theory of Hydrometallurgical Processes (Metallurgiya, Moscow, 1975).
V. V. Dolivo-Dobrovol’skii, “Some regularities of the dissolution of solids,” Zap. Leningrad. Gorn. Inst. Khim. Metallurg. Obogashch. 42 (3), 3–23 (1963).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Geidarov, A.A., Alyshanly, G.I., Gulieva, A.A. et al. Kinetic Laws of the Dissolution of Alunite from Alunite Ores with an Alkali Solution. Russ. Metall. 2020, 933–937 (2020). https://doi.org/10.1134/S0036029520090050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520090050