Abstract
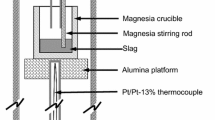
Reaction rates have been measured for the reduction of FeO from a lime-silica-alumina slag over carbon-saturated iron in graphite crucibles. The initial FeO concentrations were under 5 pet in a base slag composition of 46 to 47 pet CaO, 38 to 39 pet SiO2, 15 to 16 pet A12O8.
The reaction rate was found to be proportional to about the second power of the analyzed iron content of the slag. Two rate constants were obtained for simultaneous reactions at the slag-metal and slag-graphite interfaces. Values for these constants at 1430°C (2606°F) were k1 = 0.00058 g FeO per (min) (sq cm) (pet FeO)2 at the metal and k2 = 0.00012 (same dimensions) at the graphite. The temperature coefficient was not measured independently for the two reactions, but the net effect of a 140°C rise in temperature was small.
Iron droplets rose to the surface of the slag with gas bubbles and collected in a ring adjacent to the crucible wall in amount equal to or greater than the weight of iron calculated from FeO reduction, in those runs with both slag and metal. When only slag was present in the crucible, the iron beads were more evenly distributed over the entire crucible wall and were smaller in size and total amount.
It is not possible to deduce a reaction mechanism to interpret the observations on the basis of these experiments alone, but several alternatives have been discussed in terms of intermediate reaction products, dissociation of FeO in the slag, diffusion, nucleation of gas or metal, and surface phenomena leading to reaction through iron films on rising bubbles of CO in the slag.
The rate of reduction of FeO in the absence of sulphur is qualitatively consistent with the part that this reaction is believed to play in the desulphurization of iron by slags under similar experimental conditions.
Similar content being viewed by others
References
S. P. Kinney: Technical Paper 422 U. S. Bureau of Mines (1929) 184 pp.
T. E. Dancey: Journal Iron & Steel Inst., London (1951) 169, pp. 17–24.
G. Derge, W. O. Philbrook, and K. M. Goldman: Trans. AIME (1950) 188, pp. 1111–1119; Journal of Metals (September 1950).
W. F. Holbrook and T. L. Joseph: Trans. AIME (1936) 120, pp. 99–117.
Lo-Ching Chang and K. M. Goldman: Trans. AIME (1948) 176, pp. 309–327; Metals Technology (June 1948).
K. M. Goldman, G. Derge, and W. O. Philbrook: Trans. AIME (1954) 200, pp. 534–540; Journal of Metals (May 1954).
W. O. Philbrook, P. J. Zorena, C. W. McCoy, and J. Stasik: Unpublished research, Metals Research Laboratory, Carnegie Institute of Technology (1954).
K. J. Laidler: Textbook of Chemical Kinetics. (1950) p. 3. New York. McGraw-Hill Book Co. Inc.
M. T. Simnad, G. Derge, and I. George: Trans. AIME (1954) 200, pp. 1386–1390; Journal of Metals (December 1954).
A. E. Martin and G. Derge: Trans. AIME (1943) 154, pp. 104–115; Metals Technology (August 1943).
Basic Open Hearth Steelmaking. (1951) Revised Ed., Chap. 15. New York. AIME.
R. E. Boni and G. Derge: Trans. AIME (1956) 206, pp. 53–59; Journal of Metals (January 1956).
Author information
Authors and Affiliations
Additional information
TP 4108C. Manuscript, Jan. 3, 1955. Chicago Meeting, February 1955.
Rights and permissions
About this article
Cite this article
Philbrook, W.O., Kirkbride, L.D. Rate of FeO Reduction from a CaO-SiO2-Al2O3 Slag by Carbon-Saturated Iron. JOM 8, 351–356 (1956). https://doi.org/10.1007/BF03377696
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03377696