Abstract
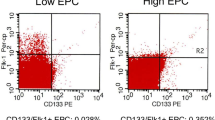
Objective: A relevant biological role of circulating endothelial progenitor cells (EPC) was recently demonstrated. EPC are generated in the bone marrow, and interact with damaged endothelium, restoring the integrity of the monolayer. Therefore, aim of the present study was to evaluate EPC in the blood of patients with untreated Graves’ hyperthyroidism (GD), in whom an increased oxidative stress was observed. Design and methods: Twenty-three patients with untreated active GD and 18 matched normal controls (NC) were included in the study. Circulating EPC were isolated from peripheral blood. Mononuclear cells were cultured with endothelial basal medium supplemented with EGM SingleQuots, and were identified by positive double staining after 7 days in culture. Circulating levels of C reactive protein, total antioxidant power, interleukin (IL)-6, IL-18, monocyte chemoattractant protein-1, tumor necrosis facotr-α, soluble vascular cell adhesion molecule (VCAM) and intracellular adhesion molecule were evaluated by enzyme-linked immunosorbent assay kit. EPC number was also evaluated in a subgroup of GD patients after restoration of euthyroidism. Results: Systolic blood pressure resulted increased in GD patients compared with control subjects whereas diastolic blood pressure was not significantly different. Patients with GD showed an increase in circulating levels of IL-18 and VCAM-1 and a reduction of total antioxidant power (p<0.05) compared to NC. Moreover, a reduced number of EPC was observed in patients with GD compared to NC (p<0.05) which turned to NC values after restoring euthyroidism. Conclusion: Patients with GD showed a reduction in the physiological protective mechanisms against endothelial damage, probably induced by increased inflammation and oxidative stress.
Similar content being viewed by others
References
Weetman AP. Graves’ disease. N Engl J Med 2000, 343: 1236–48.
Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev 1998, 19: 673–716.
Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001, 344: 501–9.
Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab 2002, 87: 968–74.
Vargas F, Moreno JM, Rodríguez-Gómez I, et al. Vascular and renal function in experimental thyroid disorders. Eur J Endocrinol 2006, 154: 197–212.
Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci 2006, 63: 414–34.
Miyauchi S, Matsuura B, Onji M. Increased levels of serum interleukin-18 in Graves’ disease. Thyroid 2000, 10: 815–9.
Rogers JS 2nd, Shane SR, Jencks FS. Factor VIII activity and thyroid function. Ann Intern Med 1982, 97: 713–6.
Baumgartner-Parzer SM, Wagner L, Reining G, et al. Increase by tri-iodothyronine of endothelin-1, fibronectin and von Willebrand factor in cultured endothelial cells. J Endocrinol 1997, 154: 231–9.
Coban E, Aydemir M, Yazicioglu G, Ozdogan M. Endothelial dysfunction in subjects with subclinical hyperthyroidism. J Endocrinol Invest 2006, 29: 197–200.
Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275: 964–7.
Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999, 85: 221–8.
Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001, 89: E1–7.
Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003, 348: 593–600.
Quyyumi AA. Circulating endothelial progenitor cells as novel biological determinants of vascular function and risk. Can J Cardiol 2004, 20(Suppl B): 44B–8B.
Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005, 111: 2981–7.
Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005, 353: 999–1007.
European Society of Hypertension-European Society of Cardiology Guidelines Committee. European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003, 21: 1011–53.
Dimmeler S, Aicher A, Vasa M,et al. HMG-CoAreductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 2001, 108: 391–7.
Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonist. Hypertension 2005, 45: 526–9.
Cappelli C, Pirola I, De Martino E, et al. The role of imaging in Graves’ disease: a cost-effectiveness analysis. Eur J Radiol 2008, 65: 99–103.
Napoli R, Biondi B, Guardasole V, et al. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation 2001, 104: 3076–80.
Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 1995, 75: 519–60.
Cunninghan K, Gotlieb A. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 2005, 85: 9–23.
Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T. Features of endothelial dysfunction in early diabetic nephropathy. Lancet 1989, 1: 461–3.
Kario K, Matsuo T, Kobayashi H, Matsuo M, Sakata T, Miyata T. Activation of tissue factor-induced coagulation and endothelial cell dysfunction in non-insulin-dependent diabetic patients with microalbuminuria. Arterioscler Thromb Vasc Biol 1995, 15: 1114–20.
Delva P, Degan M, Vallerio P, et al. Endothelial progenitor cells in patients with essential hypertension. J Hypertens 2007, 25: 127–32.
Pirro M, Schillaci G, Menecali C, et al. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34 cells of hypertensive patients. J Hypertens 2007, 25: 2093–9.
Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med 2008, 102: 1073–9.
Carneiro-Ramos MS, Silva VB, Santos RA, Barreto-Chaves ML. Tissue-specific modulation of angiotensin-converting enzyme (ACE) in hyperthyroidism. Peptides 2006, 27: 2942–9.
Barreto-Chaves ML, Carrillo-Sepúlveda MA, Carneiro-Ramos MS, Gomes DA, Diniz GP. The crosstalk between thyroid hormones and the Renin-Angiotensin System. Vascul Pharmacol 2010, 52: 166–70.
Touyz RM, Schiffrin EL. Signal transduction mechanism mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 2000, 52: 639–72.
Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cells senescence through induction of oxidative stress. J Hypertens 2005, 23: 97–104.
Komosinska-Vassev K, Olczyk K, Kucharz EJ, Marcisz C, Szczotka KW, Kotulska A. Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves’ disease during therapy. Clin Chim Acta 2000, 300: 107–17.
Videla LA, Sir T, Wolff C. Increased lipid peroxidation in hyperthyroid patients: suppression by propylthiouracil treatment. Free Radic Res Commun 1988, 5: 1–10.
Magsino CH Jr, Hamouda W, Ghanim H, Browne R, Aljada A, Dandona P. Effect of triiodothyronine on reactive oxygen species generation by leukocytes, indices of oxidative damage, and antioxidant reserve. Metabolism 2000, 49: 799–803.
Torun AN, Kulaksizoglu S, Kulaksizoglu M, Pamuk BO, Isbilen E, Tutuncu NB. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin Endocrinol (Oxf) 2009, 70: 469–74.
De Bellis A, Bizzarro A, Gattoni A, et al. Behaviour of soluble intercellular adhesion molecule-1 and endothelial-leukocyte adhesion molecule-1 concentrations in patients with Graves’ disease with or without ophthalmopathy and in patients with toxic adenoma. J Clin Endocrinol Metab 1995, 80: 2118–21.
Lee WY, Suh JY, Rhee EJ, Park JS, Sung KC, Kim SW. Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lpa levels according to thyroid function status. Arch Med Res 2004, 35: 540–5.
Dong C, Crawford LE, Goldschmidt-Clermont PJ. Endothelial progenitor obsolescence and atherosclerotic inflammation. J Am Coll Cardiol 2005, 45: 1458–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Ciuceis, C., Pilu, A., Cappelli, C. et al. Decreased number of circulating endothelial progenitor cells in patients with Graves’ hyperthyroidism. J Endocrinol Invest 34, 335–339 (2011). https://doi.org/10.1007/BF03347455
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347455