Abstract
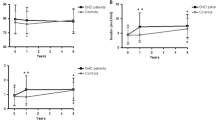
In obesity there is a clear reduction of both spontaneous and stimulated GH secretion. Furthermore, in obese patients the somatotrope responsiveness to provocative stimulation is selectively refractory to the inhibitory effect of glucose load. It has been hypothesized that hyperinsulinism of obese patients could play a role in the pathogenesis of these alterations. Aim of the present study was to verify the GH response to GHRH and the ability of glucose load to inhibit it in patients with essential hypertension in whom hyperinsulinism and insulin resistance are frequently present. To this goal, 7 patients with essential hypertension (HP, age, mean±SE: 29.6±2.4 yr, 3 females and 4 males, BMI: 21.7±1.2 kg/m2), 7 obese (OB, 4 females and 3 males, 31.9±4.1 yr, 35.6±2.0 kg/m2) and 7 normal subjects (NS, 4 females and 3 males, 28.3±3.9 yr, 21.0±1.6 kg/m2) underwent the following tests: GHRH (1 pg/kg iv at time 0) alone and preceded by oral glucose load (OGTT, 100 g po at — 45 min). Basal insulin levels were similar in HP and OB (11.3±0.5 and 12.7±2.2 µU/ml, respectively); these, in turn, were higher (p<0.005) than those in NS (6.8±0.8 µU/ml). Basal plasma glucose levels in HP were similar to those in OB and NS (80.3±3.6, 86.9±6.7 and 84.4±1.7 mg/dl, respectively). In HP and OB and NS basal GH (1.0±0.5, 1.0±0.6 and 0.3±0.1 εg/l, respectively) and IGF-I levels (132.6±14.8, 137.3±13.2 and 138.8±12.2 εg/l, respectively) were similar. In HP the GH response to GHRH (AUC: 1058.8±347.8 εg/l/min) was similar to that observed in NS (959.0±167.8 εg/l/min) and higher than that in OB (344.8±67.2 εg/l/min, p<0.01). OGTT clearly blunted (p<0.01) the GHRH-induced GH response in HP as well as in NS (401.8±104.4 and 521.6±76.6 (g/l/min, respectively) but not in OB (387.4±78.8 (g/l/min). The OGTT-induced insulin levels in HP did not differ from those of OB, both being higher (p<0.05) than those recorded in NS. Glucose levels after OGTT were similar in the three groups. In conclusion, this study demonstrates that, like in normal subjects but differently from in obese patients the GH response to GHRH is normal in patients with essential hypertension and it is normally inhibited by oral glucose load even when these patients show high insulin levels. Thus, it is unlikely that the low somatotrope secretion and its refractoriness to inhibition by glucose load in obesity is due to hyperinsulinism.
Similar content being viewed by others
References
Veldhuis J.D., Iranmanesh A., Ho K.K.Y., Waters M.J., Johnson M.L., Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J. Clin. Endocrinol. Metab. 72: 51, 1991.
Cordido F., Dieguez C., Casanueva F.F. Effect of central cholinergic neurotransmission enhancement by pyridostigmine on the growth hormone secretion elicited by Clonidine, arginine, or hy-poglicemia in normal and obese subjects. J. Clin. Endocrinol. Metab. 70: 1361, 1990.
Ghigo E., Procopio M., Boffano G.M., Arvat E., Valente F., Maccario M., Mazza E., Camanni F. Arginine potentiates but does not restore the blunted GH response to GHRH in obesity. Metabolism 41: 560, 1992.
Maccario M., Procopio M., Grottoli S., Oleandri S.E., Razzore P., Camanni F., Ghigo E. In obesity the somatotrope response to either growth hormone-releasing hormone or arginine is inhibited by somatostatin or pirenzepine but not by glucose. J. Clin. Endocrinol. Metab. 80: 3774, 1995.
Melmed S. Insulin suppresses growth hormone secretion by rat pituitary cells. J. Clin. Invest. 73: 1425, 1984
Loche S., Cappa M., Borrelli P., Faedda D., Crinó A., Cella S.G., Corda R., Muller E.E., Pintor C. Reduced growth hormone response to growth hormone-releasing hormone in children with simple obesity: evidence for somatomedin-C mediated inhibition. Clin. Endocrinol. (Oxf.) 27: 145, 1987
Grunstein H.S., James D.E., Storlien L.H., Smythe G.A., Kraegen E.W. Hyperinsulinemia suppresses glucose utilization in specific brain regions: in vivo studies using the eu-glycemic clamp in the rat. Endocrinology 116: 604, 1985.
Ferrannini E., Buzzigoli G., Bonadonna R., Giorico M.A., Oleggini M., Graziadei L., Pedrinelli R., Brandi L., Bevilacqua S. Insulin resistance in essential hypertension. N. Engl. J. Med. 6: 350, 1987.
Landsberg L. Hyperinsulinemia: possible role in obesity-induced hypertension. Hypertension (19 suppl I) 1: 61, 1992
De Fronzo R.A., Ferranini E. Insulin Resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipi-demia, and atherosclerotic cardiovascular disease. Diabetes Care 14, 3, 1991
Barbieri C., Ferrari C., Caldara R., Curtarelli G. Growth hormone secretion in hypertensive patients: evidence for a derangement in central adrenergic function. Clin. Sci. 58: 135, 1980.
Giustina A., Doga M., Bossoni S., Bodini C., Legati F., Pizzocolo G., Romanelli G. Central alpha-2 adrenergic function in patients with essential hypertension. Horm. Metab. Res., 22: 451, 1990.
Fifth Report on the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch. Intern. Med. 153: 154, 1993.
Frystyk J., Vestbo E., Skjaerbaek C., Mogensen C.E., Orskov H. Free insulin-like growth factors in human obesity. Metabolism 44: (suppl 4) 37, 1995.
Poulos J.E., Leggett-Frazier N., Khazanie P., Long S., Sportsman R., MacDonald K., Caro J.F. Circulating insulin-like growth factor I concentrations in clinically severe obese patients with and without NIDDM in response to weight loss. Horm. Metab. Res. 26: 478, 1994.
Isaacs R.E., Gardner D.G., Baxter J.D. Insulin regulation of rat growth hormone gene expression. Endocrinology 120: 2022, 1987.
Press M., Caprio S., Tamborlane W., Bhushan R., Thorner M., Vale W., Rivier J., Sherwin R. Pituitary response to growth hormone-releasing hormone in IDDM. Diabetes 41: 17, 1992.
Hansen A.P. The effect of intravenous glucose infusion on the exercise-induced serum growth hormone rise in normals and juvenile diabetics. Scand. J. Clin. Lab. Invest. 28: 195, 1971.
Masuda A., Shibasaki T., Nakahara M., Imaki T., Kiyosawa Y., Jibiki K., Demura H., Shizume K., Ling N. The effects of glucose on growth hormone (GH)-re-leasing hormone-mediated GH secretion in man. J. Clin. Endocrinol. Metab. 60: 523, 1985.
Davies R.R., Turner S., Johnston D.G. Oral glucose inhibits growth hormone secretion induced by human pancreatic growth factor 1–44 in normal man. Clin. Endocrinol. (Oxf.) 21: 477, 1984.
Ghigo E., Miola C., Aimaretti G., Valente F., Procopio M., Arvar E., Yin-Zhang W., Camanni F. Arginine abolishes the inhibitory effect of glucose on the GH response to GHRH in man. Metabolism 41: 209, 1992.
Page M.D., Kopperschaar P.F., Edwards C.A., Dieguez C., Scanion M.F. Additive effect of growth hormone releasing factor and insulin hypoglycemia on growth hormone release in man. Clin. Endocrinol. (Oxf.) 26: 589, 1987.
Grottoli S., Procopio M., Maccario M., Zini M., Oleandri S.E., Tassone F., Valcavi R., Ghigo E. In obesity, glucose load loses its early inhibitory, but maintains its late stimulatory effect on somatotrope secretion. J. Clin. Endocrinol. Metab. 82: (in press), 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Limone, P., Oleandri, S.E., Catt, P.A. et al. The inhibitory effect of glucose on growth hormone secretion is lost in obesity but not in hypertension. J Endocrinol Invest 20, 616–620 (1997). https://doi.org/10.1007/BF03346919
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03346919