Abstract
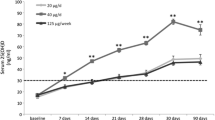
Background: Vitamin D compounds are effective in managing elevated PTH levels in secondary hyperparathyroidism (SHPT) of renal failure. However, undesired increases in serum calcium and phosphorus associated with compounds such as calcitriol [1,25(OH)2D3] has prompted a search for compounds with improved safety profiles. 1α,24(S)(OH)2D2 (1,24(OH)2D2) is a vitamin D2 metabolite with low calcium-mo bilizing activity in vivo. We studied the efficacy of 1,24(OH)2D2 in mice lacking the CYP27B1 enzyme [25-hydroxyvitamin D-1α-hydroxylase (1α-OHase)], a novel vitamin D deficiency model with SHPT. Materials and methods: 1α-OHase-deficient (−/−) mice and normal (+/−) heterozygous littermates received 1,24(OH)2D2 (100, 300, 1000, and 3000 pg/g/day) or 1,25(OH)2D3 (30, 300, and 500 pg/g/day) for 5 weeks via daily sc injection. Control groups received vehicle. Results: Vehicle-treated 1α-OHase-deficient mice were hypocalcemic and had greatly elevated serum PTH. 1,24(OH)2D2 at doses above 300 pg/g/day normalized serum calcium, serum PTH, bone growth plate morphology, and other bone parameters. No hypercalcemia was observed at any dose of 1,24(OH)2D2 in normal or 1α-OHase-deficient animals. In contrast, 1,25(OH)2D2 at only 30 pg/g/day normalized calcemia, serum PTH, and bone parameters, but at higher doses completely suppressed PTH and caused hypercalcemia in both 1α-OHase-deficient and normal mice. Treatment with 500 pg/g/day of 1,25(OH)2D3 also induced osteomalacia in normal animals. Conclusion: 1,25(OH)2D3 was maximally active at 10-fold lower doses than 1,24(OH)2D2, but induced hypercalcemia and osteomalacia at high doses. 1,24(OH)2D2 normalized serum calcium, serum PTH, and bone histomorphometry without hypercalcemia in 1α-OHase-deficient mice with SHPT.
Similar content being viewed by others
References
St-Arnaud R, Dardenne O, Glorieux FH. Etiologie moléculaire des rachitismes vitamino-dépendents héréditaires. médecine/sciences 2001, 17: 1289–96.
Hruska KA. Renal osteodystrophy. Baillieres Clin Endocrinol Metab 1997, 11: 165–94.
Slatopolsky E, Gonzalez E, Martin K. Pathogenesis and treatment of renal osteodystrophy. Blood Purif 2003, 21: 318–26.
Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000, 342: 1478–83.
Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology 2001, 142: 3135–41.
Panda DK, Miao D, Tremblay ML, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 2001, 98: 7498–503.
Dardenne O, Prudhomme J, Hacking SA, Glorieux FH, St-Arnaud R. Rescue of the pseudo-vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: biochemical, histomorphometric, and biomechanical analyses. J Bone Miner Res 2003, 18: 637–43.
Andress DL, Norris KC, Coburn JW, Slatopolsky EA, Sherrard DJ. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med 1989, 321: 274–9.
Gallieni M, Brancaccio D, Padovese P, et al. Low-dose intravenous calcitriol treatment of secondary hyperparathyroidism in hemodialysis patients. Italian Group for the Study of Intravenous Calcitriol. Kidney Int 1992, 42: 1191–8.
Slatopolsky E, Dusso A, Brown AJ. Control of uremic bone disease: role of vitamin D analogs. Kidney Int Suppl 2002: 143–8.
Brown AJ, Finch J, Takahashi F, Slatopolsky E. Calcemic activity of 19-Nor-1,25(OH)(2)D(2) decreases with duration of treatment. J Am Soc Nephrol 2000, 11: 2088–94.
Frazão JM, Chesney RW, Coburn JW. Intermittent oral 1alpha-hydroxyvitamin D2 is effective and safe for the suppression of secondary hyperparathyroidism in haemodialysis patients. 1alphaD2 Study Group. Nephrol Dial Transplant 1998, 13 (Suppl 3): 68–72.
Frazão JM, Elangovan L, Maung HM, et al. Intermittent doxercalciferol (1alpha-hydroxyvitamin D(2)) therapy for secondary hyperparathyroidism. Am J Kidney Dis 2000, 36: 550–61.
Martin KJ, González EA, Gellens M, Hamm LL, Abboud H, Lindberg J. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 1998, 9: 1427–32.
Maung HM, Elangovan L, Frazão JM, et al. Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1alpha-hydroxyvitamin D(2)) in dialysis patients with secondary hyperparathyroidism: a sequential comparison. Am J Kidney Dis 2001, 37: 532–43.
Knutson JC, LeVan LW, Valliere CR, Bishop CW. Pharmacokinetics and systemic effect on calcium homeostasis of 1 alpha,24-dihydroxyvitamin D2 in rats. Comparison with 1 alpha,25-dihydroxyvitamin D2, calcitriol, and calcipotriol. Biochem Pharmacol 1997, 53: 829–37.
Strugnell S, Byford V, Makin HL, et al. 1 alpha,24(S)-dihydroxyvitamin D2: a biologically active product of 1 alpha-hydroxyvitamin D2 made in the human hepatoma, Hep3B. Biochem J 1995, 310 (Pt 1): 233–41.
Dickson GR. Methods of calcified tissue preparation. New York: Elsevier. 1984.
Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 1987, 2: 595–610.
Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). Bone 2003, 32: 332–40.
Ohyama Y, Noshiro M, Okuda K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett 1991, 278: 195–8.
Hoenderop JG, van der Kemp AW, Urben CM, Strugnell SA, Bindels RJ. Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. Kidney Int 2004, 66: 1082–9.
Brown AJ, Ritter CS, Holliday LS, Knutson JC, Strugnell SA. Tissue distribution and activity studies of 1,24-dihydroxyvitamin D2, a metabolite of vitamin D2 with low calcemic activity in vivo. Biochem Pharmacol 2004, 68: 1289–96.
Tenenhouse HS, Gauthier C, Chau H, St-Arnaud R. 1alpha-Hydroxylase gene ablation and Pi supplementation inhibit renal calcification in mice homozygous for the disrupted Npt2a gene. Am J Physiol Renal Physiol 2004, 286: F675–81.
Omdahl JL, Bobrovnikova EA, Choe S, Dwivedi PP, May BK. Overview of regulatory cytochrome P450 enzymes of the vitamin D pathway. Steroids 2001, 66: 381–9.
Zinser GM, Tribble E, Valrance M, et al. 1,24(S)-dihydroxyvitamin D2, an endogenous vitamin D2 metabolite, inhibits growth of breast cancer cells and tumors. Anticancer Res 2005, 25: 235–41.
Takahashi F, Finch JL, Denda M, Dusso AS, Brown AJ, Slatopolsky E. A new analog of 1,25-(OH)2D3, 19-NOR-1,25-(OH)2D2, suppresses serum PTH and parathyroid gland growth in uremic rats without elevation of intestinal vitamin D receptor content. Am J Kidney Dis 1997, 30: 105–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
St-Arnaud, R., Arabian, A., Yu, V.W.C. et al. 1α,24(S)(OH)2D2 normalizes bone morphology and serum parathyroid hormone without hypercalcemia in 25-hydroxyvitamin D-1-hydroxylase (CYP27B1)-deficient mice, an animal model of vitamin D deficiency with secondary hyperparathyroidism. J Endocrinol Invest 31, 711–717 (2008). https://doi.org/10.1007/BF03346420
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03346420