Abstract
Summary
Rationale: Calcidiol can be employed to correct vitamin D deficiency. Main results: Calcidiol administered at daily and weekly regimens over a period of 3 months was able to successfully raise 25-hydroxyvitamin D levels without altering other markers related to bone and mineral metabolism. Significance: Calcidiol supplementation is effective and safe.
Introduction
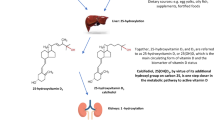
The correction of vitamin D status is necessary to maintain an optimal mineral and skeletal homeostasis. Despite cholecalciferol (vitamin D3) is the most commonly used drug for vitamin D supplementation, the more hydrophilic compound calcidiol (25-hydroxyvitamin D3) can be employed at daily, weekly, and monthly regimens to reach in the short term the target levels of serum 25-hydroxyvitamin D [25(OH)D]. In the administration of different doses of calcidiol pharmacokinetic study (ADDI-D study), the efficacy and safety of daily and weekly dosages of calcidiol were tested.
Methods
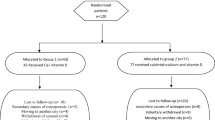
A total of 87 Caucasian, community-dwelling, postmenopausal women, aged 55 years or older, with vitamin D inadequacy (serum 25(OH)D levels <30 ng/ml, with mean 25(OH)D below 20 ng/ml, namely 16.5 ± 7.5 ng/ml) were randomized to receive three different dosages of calcidiol: 20 μg/day, 40 μg/day, and 125 μg/week for 3 months. The attained level of serum 25(OH)D was selected as primary endpoint to assess efficacy, while other parameters of mineral metabolism, (serum calcium, parathyroid hormone, phosphate, FGF23, urinary calcium, and markers of bone turnover) were assessed as secondary endpoints to establish safety.
Results
In all the three groups, serum 25(OH)D values significantly and promptly rose and plateaued above the 30 ng/ml threshold remaining within safety interval after 14 days of treatment, with similar efficacy for the similar daily and weekly dose regimens. The different dosages were also equally effective in controlling secondary hyperparathyroidism. No significant changes in calcium and phosphate metabolism and in bone turnover markers were observed for any of the treatments, confirming the safety of this compound.
Conclusions
The results of this study demonstrate the short- and mid-term efficacy and safety on core parameters of mineral metabolism of different daily or weekly dosages of calcidiol when used to treat vitamin D inadequacy or deficiency in postmenopausal women. Further studies are needed to assess falls as primary outcome of calcidiol supplementation.





Similar content being viewed by others
References
Rizzoli R, Boonen S, Brandi ML, Bruyère O, Cooper C, Kanis JA, Kaufman JM, Ringe JD, Weryha G, Reginster JY (2013) Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin 29:305–313
Rizzoli R, Stevenson JC, Bauer JM, van Loon LJ, Walrand S, Kanis JA, Cooper C, Brandi ML, Diez-Perez A, Reginster JY, Task Force ESCEO (2014) The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 79:122–132
Herrmann M, Farrell CL, Pusceddu I, Fabregat-Cabello N, Cavalier E (2017) Assessment of vitamin D status—a changing landscape. Clin Chem Lab Med 55:3–26
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443
Pepe J, Romagnoli E, Nofroni I, Pacitti MT, De Geronimo S, Letizia C, Tonnarini G, Scarpiello A, D'Erasmo E, Minisola S (2005) Vitamin D status as the major factor determining the circulating levels of parathyroid hormone: a study in normal subjects. Osteoporos Int 16:805–812
Romagnoli E, Pepe J, Piemonte S, Cipriani C, Minisola S (2013) Management of endocrine disease: value and limitations of assessing vitamin D nutritional status and advised levels of vitamin D supplementation. Eur J Endocrinol 169:R59–R69
Souberbielle JC, Brazier F, Piketty ML, Cormier C, Minisola S, Cavalier E (2017) How the reference values for serum parathyroid hormone concentration are (or should be) established? J Endocrinol Investig 40:241–256
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B (2004) Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639
Bischoff-Ferrari HA, Willett WC, Orav EJ, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B (2012) A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 367:40–49
Carmeliet G, Dermauw V, Bouillon R (2015) Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab 29:621–631
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A (2016) Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 176:175–183
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2012) Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab 97:1153–1158
Brandi ML, Minisola S (2013) Calcidiol [25(OH)D3]: from diagnostic marker to therapeutical agent. Curr Med Res Opin 29:1565–1572
Cianferotti L, Cricelli C, Kanis JA, Nuti R, Reginster JY, Ringe JD, Rizzoli R, Brandi ML (2015) The clinical use of vitamin D metabolites and their potential developments: a position statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine 50:12–26
Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoeklin E, Goessl R, Henschkowski J, Bischoff-Ferrari HA (2014) Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone 59:14–19
Bischoff-Ferrari HA, Dawson-Hughes B, Stocklin E, Sidelnikov E, Willett WC, Edel JO, Stähelin HB, Wolfram S, Jetter A, Schwager J, Henschkowsi J, von Eckardstein A, Egli A (2012) Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res 27:160–169
Cashman KD, Seamans KM, Lucey AJ, Stöcklin E, Weber P, Kiely M, Hill TR (2012) Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr 95:1350–1356
Cipriani C, Romagnoli E, Pepe J, Russo S, Carlucci L, Piemonte S, Nieddu L, McMahon DJ, Singh R, Minisola S (2013) Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab 98:2709–2715
Vieth R (1999) Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 69:842–856
Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC (2009) Benefit-risk assessment of vitamin D supplementation. Osteoporos Int 21:1121–1132
Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C (2007) Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 46:1852–1857
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303:1815–1822
Rossini M, Gatti D, Viapiana O, Fracassi E, Idolazzi L, Zanoni S, Adami S (2012) Short-term effects on bone turnover markers of a single high dose of oral vitamin D3. J Clin Endocrinol Metab 97:E622–E626
Rossini M, Adami S, Viapiana O, Fracassi E, Idolazzi L, Povino MR, Gatti D (2012) Dose-dependent short-term effects of single high doses of oral vitamin D(3) on bone turnover markers. Calcif Tissue Int 91:365–369
Smith LM, Gallagher JC, Suiter C (2017) Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2017.03.015
Haddad JG Jr, Rojanasathit S (1976) Acute administration of 25-hydroxycholecalciferol in man. J Clin Endocrinol Metab 42:284–290
Russo S, Carlucci L, Cipriani C, Ragno A, Piemonte S, Fiacco RD, Pepe J, Fassino V, Arima S, Romagnoli E, Minisola S (2011) Metabolic changes following 500 μg monthly administration of calcidiol: a study in normal females. Calcif Tissue Int 89:252–257
Lawson DE, Sedrani SH, Douglas J (1986) Interrelationships in rats of tissue pools of cholecalciferol and 25-hydroxycholecalciferol formed in u.v. light. Biochemist J233:535–540
Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M (2014) Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol 144 Pt A:132–137
Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW (2008) 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 87:1738–1742
Davies M, Mawer EB, Krawitt EL (1980) Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut 21:287–292
Michaud J, Naud J, Ouimet D, Demers C, Petit J-L, Leblond FA, Bonnardeaux A, Gascon-Barré M, Michette V (2010) Reduced hepatic synthesis of calcidiol in uremia. J Am Soc Nephrol 21:1488–1497
Gonnelli S, Rossi S, Montomoli M, Caffarelli C, Cuda C, Lazzeri G, Giacchi M, Nuti R (2009) Accuracy of different reduced versions of a validated food-frequency questionnaire in Italian men and women. Calcif Tissue Int 85:221–227
Cianferotti L, Parri S, Gronchi G, Rizzuti C, Fossi C, Black DM, Brandi ML (2015) Changing patterns of prescription in vitamin D supplementation in adults: analysis of a regional dataset. Osteoporos Int 26:2695–2702
Gallagher JC, Sai A, Templin T 2nd, Smith L (2012) Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 156:425–437
Fuleihan G-H, Bouillon R, Clarke B, Chakhtoura M, Cooper C, McClung M, Singh RJ (2015) Serum 25-hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res 30:1119–1133
Blau JE, Collins MT (2015) The PTH-vitamin D-FGF23 axis. Rev Endocr Metab Disord 16:165–174
Han X, Quarles LD (2016) Multiple faces of fibroblast growth factor-23. Curr Opin Nephrol Hypertens 25:333–342
Meyer O, Dawson-Hughes B, Sidelnikov E, Egli A, Grob D, Staehelin HB, Theiler G, Kressig RW, Simmen HP, Theiler R, Bischoff-Ferrari HA (2015) Calcifediol versus vitamin D3 effects on gait speed and trunk sway in young postmenopausal women: a double-blind randomized controlled trial. Osteoporos Int 26:373–381
Acknowledgments
This work was supported though a dedicated grant from Bruno Farmaceutici (to Maria Luisa Brandi and Salvatore Minisola). We are indebted to Guido Fedele for the statistical analyses of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Luisella Cianferotti, Piergianni Biondi, Caterina Fossi, Francesco Franceschelli, Francesca Giusti, Gigliola Leoncini, and Jessica Pepe declare that they have no conflict of interest.
Salvatore Minisola served as speaker for Abiogen, Amgen, Bruno Farmaceutici, Diasorin, Eli Lilly, Italfarmaco, Fujii, and Merck Sharp & Dohme, Takeda. He served in advisory board of Amgen, Eli Lilly, and Merck Sharp & Dohme and received paid consultancy from Bruno Farmaceutici.
Heike A. Bischoff-Ferrari contributed as invited speaker and on advisory boards for Roche, Pfizer, Sanofi, DSM Nutritional Products, Nestlé, and WILD. She received investigator initiated and independent funding from DSM Nutritional Products, Roche Diagnostics, Pfizer, and Nestlé.
Maria Luisa Brandi has received consultancy fees and grant support from Alexion, Abiogen, Amgen, Eli Lilly, and Shire.
Electronic supplementary material
Supplementary table 1
(DOCX 25 kb).
Supplementary table 2
(DOCX 15 kb).
Rights and permissions
About this article
Cite this article
Minisola, S., Cianferotti, L., Biondi, P. et al. Correction of vitamin D status by calcidiol: pharmacokinetic profile, safety, and biochemical effects on bone and mineral metabolism of daily and weekly dosage regimens. Osteoporos Int 28, 3239–3249 (2017). https://doi.org/10.1007/s00198-017-4180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4180-3