Abstract
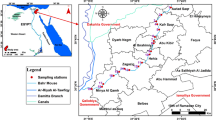
In the present study sediment and water samples collected from Kowsar Dam reservoir in Kohkiluye and Boyerahmad Province, southwest of Iran, are subjected to bulk digestion and chemical partitioning. The concentrations of nickel, lead, zinc, copper, cobalt, cadmium, manganese and iron in water and bed sediment were determined by atomic absorption spectrometry. The concentrations of metals bounded to five sedimentary phases were estimated. On this basis, the proportions of natural and anthropogenic elements were calculated.The anthropogenic portion of elements are as follows: zinc (96 %)> cobalt (88 %)> iron (78 %)> magnesium (78 %)> nickel (78 %)> copper (66 %)> lead (63 %)> cadmium (59 %). The results show sediment contamination by nickel, cadmium and lead, according to the world aquatic sediments and mean earth crust values. Manganese and copper have strong association with organic matter and are of high portion of sulfide bounded ions. Finally, The degree of sediment contamination was evaluated using enrichment factor, geo-accumulation index (Igeo) and pollution index (IPoll). The sediments were identified to be of high cadmium and lead pollution index. The pattern of pollution intensity according to enrichment factor is as follows; manganese (1.25) < copper (1.63) < zinc (1.93) < cobalt (2.35) < nickel (3.83) < lead (12.63) < cadmium (78.32). Cluster analysis was performed in order to assess heavy metal interactions between water and sediment. Accordingly, nickel, cadmium and copper are earth originated. Zinc, copper and manganese are dominated by pH. All the elemental concentrations in water and sediment are correlated except for sedimental copper.
Similar content being viewed by others
References
Al-Juboury, A. I., (2009). Natural pollution by some heavy metals in the Tigris River, northern Iraq. Int. J. Environ. Res., 3(2), 189–198 (10 pages).
Asaah, V. A.; Abimbola, A. F.; Suh, C. E., (2005). Heavy metal concentrations and distribution in surface soils of the Bassa Industrial Zone 1, Doula, Cameroon. J. Sci. Eng., 31(2A), 147–158 (12 pages).
Barretoo, W. J.; Ishikawa, D. N.; Scarminio, I. S.; Costa, J. S., (2008). Fe, Mn, P and S speciation in sediments from the Capivara Hydroelectric dam lake (Brazil) as an indicator of anthropogenic influences. Clean Soil, Air, Water, 36(4), 353–359 (7 pages).
Biati, A.; Karbassi, A. R.; Hassani, A. H.; Monavari, S. M.; Moattar, F., (2010). Role of metal species in flocculation rate during estuarine mixing. Int. J. Environ. Sci. Tech., 7(2), 327–336 (10 pages).
Bowen, H. J. M., (1979). Environmental chemistry of the elements, Academic Press, London, 333.
Broadley, M. R.; White, P. J.; Hammond, J. P.; Zelko, I.; Lux, A., (2007). Zinc in plants. New Phytol., 173(4), 677–702 (26 pages).
Carver, G. A., (1972). Glacial geology of the mountain lakes wilderness and adjacent parts of the cascade range, Oregon. Ph.D. Dissertation, University of Washington, Seattle.
Chapman, P. M., (2000). The sediment quality triad: Then, now and tomorrow. Int. J. Environ. Poll., 13(1–6), 351–356 (6 pages).
Davis, J. B., (1973). Statistic and data analysis in geology, Wiley International, New York, 456–473 (18 pages).
De, A. K., (1987). Environmental chemistry, Wiley Eastern Ltd., New Delhi.
Forstner, U., (1985). Chemical forms and reactivities of metal in sediments, in: Leschber, R.; Davis, E. D.; Hermite, P. L., Elsevir (Eds.), London, 1–30 (31 pages).
Forstner, U.; Ahlf, W.; Calmano, W.; Kersten, M., (1990). Sediment criteria development, In: Helling, D.; Rothe, P.; Forstner, U.; Stoffers, P. (Eds.), sediments and environmental geochemistry: selected aspects and case histories, Berlin, 311–338 (28 pages).
Gibs, R. J., (1973). Mechanism of trace metal transport in rivers. Sci., 180(4081), 71–73 (3 pages).
Harrison, R. J.; Dunin-Borkowski, R. E.; Putnis, A., (2002). Direct imaging of nanoscale magnetic interactions in minerals. Proc. Natl. Acad. Sci., 99(26), 16556–16561 (6 pages).
Harte, J.; Holdren, C.; Schneider, R.; Shirley, C., (1991). Toxics A to Z, a guide to everyday pollution hazards, University of California Press, Oxford, 478.
Horowitz, A. J.; Meybeck, M.; Idlafkih, Z.; Biger, F., (1999). Variations in trace element geochemistry in the Seine river basin based on floodplain deposits and bed sediments. Hydrol. Processes, 13(9), 1329–1340 (12 pages).
Huu, H. H.; Rudy, S.; Damme, A. V., (2010). Distribution and contamination status of heavy metals in estuarine sediments near Cau Ong harbor, Ha Long Bay. Vietnam, Geol. Belgica, 13(1–2), 37–47 (11 pages).
Jain, C. K.; Singhal, D. C.; Sharma, U. K., (2005). Metal pollution assessment of sediment and water in the river Hindon, India. Environ. Monit. Assess., 105(1–3), 193–207 (15 pages).
Jiao, Y.; Grant, C. A.; Bailey, L. D., (2004). Effects of phosphorus and zinc fertilizer on cadmium uptake and distribution in flax and durum wheat. J. Sci. Food Agr., 84(8), 777–785 (9 pages).
Karbassi, A. R; Monavari, S. M.; Nabi Bidhendi, Gh. R.; Nouri, J., (2008). Metal pollution assessment of sediment and water in the Shur River. Environ. Monit. Assess., 147(1–3), 107–116 (10 pages).
Kho, F. W. L.; Law, P. L.; Lai, S. H.; Oon, Y. W.; Ngu, L. H.; Ting, H. S., (2009). Quantitative dam break analysis on a reservoir earth dam. Int. J. Environ. Sci. Tech., 6(2), 203–210 (8 Pages).
Kirk, R. E.; Othmer, D. F., (1985). Kirk-Othmer Concise Encyclopedia of Chemical Technology, Wiley, New York.
Lascelles, K.; Morgan, L. G.; Nicholls, D.; Beyersmann, D., (2005). “Nickel Compounds” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim.
Lide, D. R., (1998). Handbook of Chemistry and Physics (87 ed.), CRC Press, Boca Raton, FL.
Martin, J. M.; Meybeck, M., (1979). Elemental mass-balance of material carried by major world rivers, Mar. Chem., 7(3), 173–206 (34 pages).
Mediolla, L. L.; Domingues, M. C. D.; Sandoval, M. R. G., (2008). Environmental Assessment of an Active Tailings Pile in the State of Mexico (Central Mexico), Res. J. Environ. Sci., 2(3), 197–208 (12 pages).
Opuene, K.; Okafor, E. C.; Agbozu, I. E., (2008). Partitioning characteristics of heavy metals in a non-tidal freshwater ecosystem. Int. J. Environ. Res., 2(3), 285–290 (6 pages).
Priju, C. P.; Narayana, A. C., (2007). Heavy and Trace Metals in Vembanad Lake Sediments. Int. J. Environ. Res., 1(4), 280–289 (10 pages).
Rayner-Canham, G.; Overton, T., (2003). Descriptive Organic Chemistry, Macmillan.
Rosental, R.; Eagle, G. A.; Orren, M. Y., (1986). Trace metal distribution in different chemical fractions of near shore marine sediments. Estuarine Coastal Shelf Sci., 22(3), 303–324 (22 pages).
Samans, C. H., (1949). Engineering Metals and their Alloys, MacMillan.
Schuurmann, G.; Market, B., (1998). Ecotoxicology, ecological fundamentals, chemical exposure, and biological effects. Wiley, New York.
Stamatis, N.; Kamidis, N.; Sylaios, G., (2006). Sediment and suspended matter lead contamination in the gulf of Kavala, Greece. Environ. Monit. Assess., 115(1–3), 433–449 (17 pages).
Sutherland, R. A., (2000). Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol., 39(6), 611–627 (17 pages).
Taghinia Hejabi, A.; Basavarajappa, H.T.; Qaid Saeed, A. M., (2010). Heavy metal pollution in Kabini river sediments. Int. J. Environ. Res., 4(4), 629–636 (8 pages).
Turekian, K. K.; Wedepohl, K. H., (1961). Distribution of the elements in some major units of earth’s crust. Bull. Geol. Soc. Am., 72(2), 175–192 (18 pages).
Van de Guchte, C., (1992). The sediment quality triad:an integrated approach to assess contaminated sediments, in: P. J. Newman, M. A. Piavaux, R. A. Sweeting, (Eds.), River water quality, ecological assessment and control, Brussels, 417–423 (7 pages).
Wiberg, V. E.; Wiberg, N.; Holleman, A. F., (2001). Inorganic Chemistry, Academic Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karbassi, A.R., Torabi, F., Ghazban, F. et al. Association of trace metals with various sedimentary phases in dam reservoirs. Int. J. Environ. Sci. Technol. 8, 841–852 (2011). https://doi.org/10.1007/BF03326267
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03326267