Summary
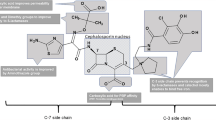
At present, desacetylcefotaxime represents the only major metabolite from a third generation cephalosporin that attains and maintains effective antimicrobial plasma and tissue concentrations following therapeutic administration of its parent drug (i.e. cefotaxime). Therapeutic concentrations of desacetylcefotaxime are produced in neonates, infants, children and adults, although because of normal developmental changes in renal function, the plasma clearance of the drug is reduced in neonates and young infants. Previous investigations of the pharmacokinetics of desacetylcefotaxime have not only demonstrated effective penetration into various tissues and fluids, but also that alterations in plasma protein concentration and/or binding do not appear to alter either the pharmacokinetics or pharmacodynamics of the drug. Consequent to a more prolonged elimination half-life for desacetylcefotaxime (e.g. approximately 1.6h in adults, 2.1h in infants and children, 9.4h in neonates) as compared to its parent drug, desacetylcefotaxime persists in plasma at concentrations which exceed the minimum concentration inhibiting 50% of organisms (MIC50) for many common paediatric pathogens for 6 to 8 hours following a cefotaxime dose. This property, coupled with excellent stability against many types of β-lactamases, produces an additive and/or synergistic antimicrobial effect when cefotaxime is used to treat infections caused by many common pathogens. Accordingly, the unique pharmacokinetic and pharmacodynamic properties of desacetylcefotaxime enable, in part, an enhanced therapeutic profile for its parent drug which may permit longer dosing intervals for cefotaxime (i.e. every 8 or 12h) to be used without necessarily compromising efficacy associated with conventional (i.e. every 6h) dosing regimens.
Similar content being viewed by others
References
Adriono T, Susanta I, Makmuri J, Santosa G. Acute mediastinitis. Paediatrica Indonesia 29: 116–120, 1989
Aldridge KE, Weeks LS, Stratton CW, Sanders CV. Comparison of the bactericidal activity of cefotaxime and desacetylcefotaxime alone and in combination against Bacteroides fragilis group organisms. Diagnostic Microbiology and Infectious Disease 12: 165–170, 1989
American Academy of Pediatrics. Report of the Committee on Infectious Diseases, 22nd ed., American Academy of Pediatrics, Elk Grove Village, Illinois, 1991
Asami K, Ohlashiki K, Ohmichi H. The influences of liver diseases on cefotaxime and cefoperazone drug movements. Japanese Journal of Clinical Pharmacology and Therapeutics 19: 37–38, 1988
Baird-Lambert J, Doyle PE, Thomas D, Cvejic M, Buchanan N. Pharmacokinetics of cefotaxime in neonates. Journal of Antimicrobial Chemotherapy 13: 471–477, 1984
Besunder JB, Reed MD, Blumer JL. Principles of drug biodisposition in the neonate: a critical evaluation of the pharmacokinetic-pharmacodynamic interface. I. Clinical Pharmacokinetics 14: 189–216, 1988
Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS. Cefotaxime: a review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 25: 223–289, 1983
Chamberlain J, Coombes JD, Dell D, Fromson JM, Ings RJ, et al. Metabolism of cefotaxime in animals and man. Journal of Antimicrobial Chemotherapy 6 (Suppl. A): 69–78, 1980
Chin NX, Neu HC. Cefotaxime and desacetylcefotaxime: an example of advantageous antimicrobial metabolism. Diagnostic Microbiology and Infectious Disease 2: 21S–31S, 1984
Coombes JD. Metabolism of cefotaxime in animals and humans. Reviews of Infectious Diseases 4 (Suppl.): 325–332, 1982
Crooks J, White LO, Burville LJ, Speidel BD, Reeves DS. Pharmacokinetics of cefotaxime and desacetylcefotaxime in neonates. Journal of Antimicrobial Chemotherapy 14 (Suppl. B): 97–101, 1984
Devlin HR, Boskovski L. The synergistic effect of cefotaxime and desacetylcefotaxime against clinical isolates of anaerobic bacteria. Drugs 35 (Suppl. 2): 45–50, 1988
Doyle PE, Jager-Roman E, Baird-Lambert J, Cvejic M, Buchanan N. Effect of prenatal exposure to betamethasone on metronid-azole elimination in premature infants. (Letter.) Journal of Pediatrics 101: 647, 1982
Drusano GL. Role of pharmacokinetics in the outcome of infections. Antimicrobial Agents and Chemotherapy 32: 289–297, 1988
Fick RB, Alexander MR, Prince RA, Kasik JE. Penetration of cefotaxime into respiratory secretions. Antimicrobial Agents and Chemotherapy 31: 815–817, 1987
Fraise AP, Meeks ACG, Richard JE. Meningitis due to Haemophilus influenzae resistant to ampicillin and chloramphenicol. Archives of Diseases of Childhood 61: 1134–1135, 1986
Friis H. Antibacterial activity of cefotaxime, desacetylcefotaxime, and the combination of the two. Diagnostic Microbiology and Infectious Disease 12: 67–72, 1989
Fuchs PC, Jones RN, Barry AL. Evaluation of disk susceptibility testing of cefotaxime/desacetylcefotaxime. Diagnostic Microbiology and Infectious Disease 12: 81–85, 1989b
Fuchs PC, Jones RN, Barry AL, Allen SD, Ayers LW, et al. Desacetylcefotaxime — another broad spectrum cephalosporin? Journal of Antimicrobial Chemotherapy 23: 165–167, 1989a
Gamier JM, Giraud F. Cefotaxime in childhood infections. Nouvelle Presse Médicale 10: 629–633, 1981
Gekle D, Rangoonwala R, Seeger K. Clinical and kinetic studies on cefotaxime in the treatment of bacterial infections in children. Infection 8 (Suppl. 4): S509–S512, 1980
Gouyon JB, Pechinot A, Safran C, Chretien P, Sandre D, et al. Pharmacokinetics of cefotaxime in preterm infants. Developmental Pharmacology and Therapeutics 14: 29–34, 1990
Graninger W, Uihlein M, Ferenci P, Moser C, Georgopoulos A. Cefotaxime and desacetylcefotaxime blood levels in hepatic dysfunction. Journal of Antimicrobial Chemotherapy 14 (Suppl. B): 143–146, 1984
Hasegawa H, Takahashi K, Imada A, Horiuchi A. Pharmacokinetics of cefotaxime and desacetylcefotaxime in renal failure patients undergoing continuous arteriovenous haemofiltration. Drugs 35 (Suppl. 2): 78–81, 1988
Helwig H. Experience with cefotaxime in children. Infection 8 (Suppl. 4): S506–S508, 1980
Humbert G, Leroy A, Nair SR, Cherubin CE. Concentrations of cefotaxime and the desacetyl metabolite in serum and CSF of patients with meningitis. Journal of Antimicrobial Chemotherapy 13: 487–494, 1984
Ings RM, Fillastre J-P, Godin M, Leroy A, Humbert G. The pharmacokinetics of cefotaxime and its metabolites in subjects with normal and impaired renal function. Reviews of Infectious Diseases 4 (Suppl.): S379–S391, 1982
Ings RMJ, Reeves DS, White LO, Bax RP, Bywater MJ, et al. The human pharmacokinetics of cefotaxime and its metabolites and the role of renal tubular secretion on their elimination. Journal of Pharmacokinetics and Biopharmaceutics 13: 121–142, 1985
Jacobs RF, Kearns GL. Cefotaxime and desacetylcefotaxime in neonates and children: a review of microbiologic, pharmacokinetic, and clinical experience. Diagnostic Microbiology and Infectious Disease 12: 93–99, 1989
Jacobs RF, Wells TG, Steele RW, Tamauchi T. A prospective randomised comparison of cefotaxime vs ampicillin and chloramphenicol for bacterial meningitis in children. Journal of Pediatrics 107: 129–133, 1985
Jager-Roman E, Doyle PE, Thomas D, Baird-Lambert J, Cvegic M, et al. Increased theophylline metabolism in premature infants after prenatal betamethasone administration. Developmental Pharmacology and Therapeutics 5: 127–135, 1982
Jenkins SG. Activity of cefotaxime/desacetylcefotaxime with two aminoglycosides against gram-negative pathogens: an example of interactive synergy. Diagnostic Microbiology and Infectious Disease 12: 51–55, 1989
Jones RN. A review of cephalosporin metabolism: a lesson to be learned for future chemotherapy. Antimicrobic Newsletter 4: 69–76, 1987
Jones RN. A review of cephalosporin metabolism: a lesson to be learned for future chemotherapy. Diagnostic Microbiology and Infectious Disease 12: 25–31, 1989
Jones RN, Barry AL. Antimicrobial activity of ceftriaxone, cefotaxime, desacetylcefotaxime, and cefotaxime-desacetylcefotaxime in the presence of human serum. Antimicrobial Agents and Chemotherapy 31: 818–820, 1987
Jones RN, Barry AL, Packer RR. The activity of cefotaxime and desacetylcefotaxime alone and in combination against anaerobes and staphylococci. Diagnostic Microbiology and Infectious Disease 2: 37S–46S, 1984
Jones RN, Barry AL, Thornsberry C. Antimicrobial activity of desacetylcefotaxime alone and in combination with cefotax-ime: evidence of synergy. Reviews of Infectious Diseases 4 (Suppl.): 366–373, 1982
Jones RN, Wilson HW. Comparative beta-lactamase hydrolysis of and inhibition by 7-aminothiazolyl alpha-methoxyimino cephalosporins. Infection 10: 303, 1982a
Jones RN, Wilson HW. The beta-lactamase hydrolysis and inhibition of cephalothin cephapirin and cefotaxime compared to their desacetyl-metabolites. Chemoterapia 1: 73, 1982b
Kearns GL, Jacobs RF, Thomas BR, Darville TL, Trang JM. Cefotaxime and desacetylcefotaxime pharmacokinetics in very low birth weight neonates. Journal of Pediatrics 114: 461–467, 1990
Kearns GL, Reed MD. Clinical pharmacokinetics in infants and children: a reappraisal. Desacetylcefotaxime pharmacokinetics in very low birth weight neonates. Clinical Pharmacokinetics 17 (Suppl. 1): 29–67, 1989
Kearns GL, Young RA, Jacobs RF. Cefotaxime dosing in infants and children: pharmacokinetic and clinical rationale for an extended dosing interval. Clinical Pharmacokinetics 4: 284–297, 1992
Lassman HB, Coombes JD. Metabolism of cefotaxime: a review. Diagnostic Microbiology and Infectious Disease 2: 3S–12S, 1984
Limbert M, Seiber G, Schrinner E. The cooperation of cefotaxime and desacetylcefotaxime with respect to antibacterial activity and beta-lactamase stability. Infection 10: 97, 1982
Macdonald CM, Fromson JM, McDonald A, Dell D, Chamberlain J, et al. Disposition of cefotaxime in rat, dog and man. Arzneimittel-Forschung 34: 1719–1723, 1984
Matsuoka T, Ota H, Takeda J, Takatani O. Basic and clinical studies of cefotaxime. Japanese Journal of Antibiotics 29: 726–732, 1986
Matzke GR, Abraham PA, Halstenson CE, Keane WF. Cefotaxime and desacetyl cefotaxime kinetics in renal impairment. Clinical Pharmacology and Therapeutics 38: 31–36, 1985
McCracken GH, Threlkeld NE, Thomas ML. Pharmacokinetics of cefotaxime in newborn infants. Antimicrobial Agents and Chemotherapy 21: 683–684, 1982
Morselli PL. Clinical pharmacology of the perinatal period and early infancy. Clinical Pharmacokinetics 17 (Suppl. 1): 13–28, 1989
Neu HC. The pharmacokinetics of new cephalosporins: significance in clinical practice. Bulletin of the New York Academy of Medicine 60: 327–339, 1984
Neu HC. Antibacterial activity of desacetylcefotaxime alone and in combination with cefotaxime. Reviews of Infectious Diseases 4 (Suppl.): 374–378, 1982
Neu HC. Pathophysiologic basis for the use of third-generation cephalosporins. American Journal of Medicine 88 (Suppl. 4A): 3S–11S, 1990
Odio CM, McCracken GH. A multicenter comparative trial of Claforan (CTX) dosed at Q 6hr vs 8hr intervals in the treatment of bacterial meningitis in infants and children. Abstract 501. Proceedings of the 17th International Congress of Chemotherapy, Berlin, FRG, 1991
Ohkawa M, Okasho A, Motoi I, Togunaga S, Shoda R, et al. Elimination kinetics of cefotaxime and desacetylcefotaxime in patients with renal insufficiency and during hemodialysis. Chemotherapy (Basel) 29: 4–12, 1983
Oizumi K, Hayashi I, Anouma S, Konno K. In vitro activity of desacetylcefotaxime and the interaction with its parent compound, cefotaxime. Drugs 35 (Suppl. 2): 57–61, 1988
Perl TM, Pfaller MA, Houston A, Wenzel RP. Effect of serum on the in vitro activities of 11 broad-spectrum antibiotics. Antimicrobial Agents and Chemotherapy 34: 2234–2239, 1990
Peterson LR, Gerding DN. Influence of protein binding of antibiotics on serum pharmacokinetics and extravascular penetration: clinically useful concepts. Reviews of Infectious Diseases 2: 340–348, 1980
Petrikkos G, Androulakis M, Goumas P, Giamarellou H. A comparative study of cefoxitin, cefotaxime, moxalactam and aztreonam kinetics in saliva. Chemioterapia 6: 355–358, 1987
Pils P, Schmidt P, Zazgornik J, Graninger W, Kopsa H, et al. Azlocillin and HR 756 in urosepsis and urinary tract infections with multi-resistant Pseudomonas and Proteus morganii. Deutsche Medizinische Wochenschrift 104: 66–67, 1979
Quintiliani R, Nightingale CH, Tilton R. Comparative pharmacokinetics of cefotaxime and ceftizoxime and the role of desacetylcefotaxime in the antibacterial activity of cefotaxime. Diagnostic Microbiology and Infectious Disease 2: S63–S70, 1984
Reller LB. Interaction of cefotaxime and desacetylcefotaxime against pathogenic bacteria; assessment with the serum bactericidal test. Diagnostic Microbiology and Infectious Disease 2: 55S–61S, 1984
Rivers TE, Martinez DR, Burke JM. Effect of protein binding on antimicrobial activity. Drug Intelligence and Clinical Pharmacy 22: 793–794, 1988
Scaglione F, Raichi M, Fraschini F. Serum protein binding and extravascular diffusion of methoxyimino cephalosporins. Time courses of free and total concentrations of cefotaxime and ceftriaxone in serum and pleural exudate. Journal of Antimicrobial Chemotherapy 26 (Suppl. A): 1–10, 1990
Schrinner E, Limbert M, Seeger K, Seibert G, Novick Jr WJ. The in vitro antimicrobial activity of desacetylcefotaxime compared to other related β-lactams. Diagnostic Microbiology and Infectious Disease 2: 13S–20S, 1984
Stevens DL, Bergstrom R, Gibbons A. Synergistic interaction of cefotaxime and its metabolite desacetylcefotaxime demonstrated by drug-impregnated disks. Diagnostic Microbiology and Infectious Disease 12: 73–80, 1989
Stratton CW, Kernodle DS, Eades SC, Weeks LS. Evaluation of cefotaxime alone and in combination with desacetylcefotaxime against strains of Staphylococcus aureus that produce variants of Staphylococcal β-lactamase. Diagnostic Microbiology and Infectious Disease 12: 57–65, 1989
Todd PA, Brogden RN. Cefotaxime, an update of its pharmacology and therapeutic use. Drugs 40: 608–651, 1990
Trang JM, Jacobs RF, Kearns GL, Brown AL, Wells TG, et al. Cefotaxime and desacetylcefotaxime pharmacokinetics in infants and children with meningitis. Antimicrobial Agents and Chemotherapy 28: 791–795, 1985
Wasilauskas BL. Effectiveness of cefotaxime alone and in combination with desacetylcefotaxime against Bacteroides fragilis. Diagnostic Microbiology and Infectious Disease 12: 39–43, 1989
Wise R. The clinical significance of protein binding and tissue concentrations in antimicrobial therapy. Clinical Pharmacokinetics 6: 59–68, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kearns, G.L. Desacetylcefotaxime. Drug Invest 4 (Suppl 2), 9–17 (1992). https://doi.org/10.1007/BF03258352
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258352