Abstract
Background: Cetuximab, a monoclonal antibody directed against the epidermal growth factor receptor, improves progression-free survival and overall survival in patients with metastatic colorectal cancer (mCRC). However, patients with a KRAS gene mutation do not benefit from cetuximab therapy.
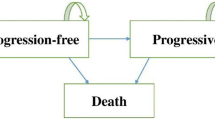
Methods: We performed a cost-effectiveness analysis of KRAS testing and cetuximab treatment as last-line therapy for patients with mCRC in Japan. In our analysis, we considered three treatment strategies. In the ‘KRAS-testing strategy’ (strategy A), KRAS testing was performed to guide treatment: patients with wildtype KRAS received cetuximab, and those with mutant KRAS received best supportive care (BSC). In the ‘no -KRAS- testing strategy’ (strategy B), genetic testing was not conducted and all patients received cetuximab. In the ‘no-cetuximab strategy’ (strategy C), genetic testing was not conducted and all patients received BSC. To evaluate the cost effectiveness of KRAS testing, the KRAS- testing strategy was compared with the no-KRAS-testing strategy; to evaluate the cost effectiveness of KRAS testing and cetuximab, the KRAS- testing strategy was compared with the no-cetuximab strategy; and to evaluate the cost effectiveness of cetuximab treatment without KRAS testing, the no-KRAS-testing strategy was compared with the no-cetuximab strategy. A three-state Markov model was used to predict expected costs and outcomes for each group. Outcomes in the model were based on those reported in a retrospective analysis of data from the National Cancer Institute of Canada Clinical Trials Group CO.17 study. We included only direct medical costs from the perspective of the Japanese healthcare payer. A 3% discount rate was used for both costs and outcome. Two outcomes, life-years (LYs) gained and quality-adjusted life-years (QALYs) gained, were used to calculate the incremental cost-effectiveness ratio (ICER).
Results: Our cost-effectiveness analysis revealed that the KRAS-testing strategy was dominant compared with the no-KRAS- testing strategy, with an expected cost reduction of ¥0.5 million per patient and an estimated budget impact of ¥3–5 billion ($US42–59 million; July 2010 values) per year. The ICER of the KRAS- testing strategy compared with the no-cetuximab strategy was ¥11 million ($US120000) per LY gained and ¥16 million ($US160 000) per QALY gained, whereas the ICER of the KRAS-testing strategy compared with the no-KRAS-testing strategy was ¥14 million ($US180 000) per LY gained and ¥21 million ($US230 000) per QALY gained. These results were supported by the sensitivity analysis.
Conclusions: KRAS testing is recommended before administering cetuximab as last-line therapy for patients with mCRC. However, our analysis suggests that the ICER of cetuximab treatment (with or without KRAS testing) is too high, even if treatment is limited to patients with wild-type KRAS.








Similar content being viewed by others
References
Ministry of Health, Labour and Welfare. Population survey report [in Japanese]. Tokyo: Ministry of Health, Labour and Welfare, 2009
Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol 2005; 23: 4553–6
Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005; 352: 476–87
deGramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47
Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229–37
Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008; 26: 2006–12
Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer: Irinotecan Study Group. N Engl J Med 2010; 343: 905–14
Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2010; 355: 1041–7
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 35: 2335–42
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26: 2013–9
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–45
Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007; 357: 2040–8
Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 2311–9
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757–65
VanCutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25: 1658–64
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 1626–34
Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010; 375: 1030–47
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008; 358: 1160–74
Messersmith WA, Ahnen DJ. Targeting EGFR in colorectal cancer. N Engl J Med 2008; 359: 1834–6
Jiang Y, Kimchi ET, Staveley-O’Carroll KF, et al. Assessment of K-ras mutation: a step toward personalized medicine for patients with colorectal cancer. Cancer 2009; 115: 3609–17
Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009; 27: 2091–6
Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine: Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1172–7
Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1253–8
Siegel JE, Weinstein MC, Russell LB, et al. Recommendations for reporting cost-effectiveness analyses: Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1339–41
Siegel JE, Torrance GW, Russell LB, et al. Guidelines for pharmacoeconomic studies: recommendations from the Panel on Cost Effectiveness in Health and Medicine. Pharmacoeconomics 1997; 11: 159–68
Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3: 419–58
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–38
Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998; 13: 397–409
Mittmann N, Au HJ, Tu D, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst 2009; 101: 1182–92
Feeny D, Furlong W, Boyle M, et al. Multi-attribute health status classification systems: Health Utilities Index. Pharmacoeconomics 1995; 7: 490–502
Japanese Society for Cancer of the Colon and Rectum. Clinical guideline for colorectal cancer [in Japanese]. Tokyo: Japanese Society for Cancer of the Colon and Rectum, 2009
Social Insurance Research Laboratory. Reimbursement schedule of social insurance and insurance [in Japanese]. Tokyo: Social Insurance Research Laboratory, 2010
Jiho, Inc. National health insurance drug price standard [in Japanese]. Tokyo: Jiho, Inc., 2010
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York: Oxford University Press, 2006
National Institute for Health and Clinical Excellence [NICE]. Guide to the methods of technology appraisal. London: NICE, 2004 [online]. Available from URL: http://www.nice.org.uk/niceMedia/pdf/TAP_Methods.pdf [Accessed 2010 Dec 6]
Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 2010; 19: 422–37
Starling N, Tilden D, White J, et al. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 2007; 96: 206–12
National Institute for Health and Clinical Excellence [NICE]. NICE technology appraisal guidance 118: bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. London: NICE, 2007 [online]. Available from URL: http://guidance.nice.org.uk/nicemedia/live/11612/33927/33927.pdf [Accessed 201 Dec 6]
Tappenden P, Jones R, Paisley S, et al. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007; 11: 1–128, iii-iv
National Institute for Health and Clinical Excellence [NICE]. NICE technology appraisal guidance 176: cetuximab for the first-line treatment of metastatic colorectal cancer. London: NICE, 2010 [online]. Available from URL: http://www.nice.org.uk/nicemedia/live/12216/45198/45198.pdf [Accessed 2010 Dec 6]
Claxton K, Briggs A, Buxton MJ, et al. Value based pricing for NHS drugs: an opportunity not to be missed? BMJ 2008; 336: 251–4
Zhang Z, Sun J. Interval censoring. Stat Methods Med Res 2010; 19: 53–7
Littlejohns P, Garner S, Doyle N, et al. 10 years of NICE: still growing and still controversial. Lancet Oncol 2009; 1: 417–24
Acknowledgments
This study was supported by Roche Diagnostics K.K. Our work was not influenced by the funder. There are no other conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The differences between the patient populations used in our analysis versus those used in the Canadian analysis[29] of the NCIC CO.17 trial[14] data are defined below. See table A-1 for details of study population subgroups (1) to (6).
Population of our Analysis
-
In the KRAS-testing strategy, patients consisted of (1) and (4);
-
in the no-KRAS-testing strategy, patients consisted of (1) and (3); and
-
in the no-cetuximab strategy, patients consisted of (2) and (4).
We compared three strategies with each other: (1) + (4)vs(1) + (3)vs(2) + (4).
Population of Canadian Analysis
-
In the entire study population, the cetuximab group consisted of (1), (3), and (5), and the BSC group consisted of (2), (4), and (6).
-
In the wild-type KRAS population, the cetuximab group was (1) and the BSC group was (2).
The Canadian analysis compared cetuximab with BSC in two populations: (1) + (3) + (5) vs (2)+ (4)+ (6), and (1) vs (2).
-
1.
Patients with wild-type KRAS treated with cetuximab.
-
2.
Patients with wild-type KRAS treated with BSC.
-
3.
Patients with mutant KRAS treated with cetuximab.
-
4.
Patients with mutant KRAS treated with BSC.
-
5.
Patients with unknown KRAS status treated with cetuximab.
-
6.
Patients with unknown KRAS status treated with BSC.
Rights and permissions
About this article
Cite this article
Shiroiwa, T., Motoo, Y. & Tsutani, K. Cost-Effectiveness Analysis of KRAS Testing and Cetuximab as Last-Line Therapy for Colorectal Cancer. Mol Diag Ther 14, 375–384 (2010). https://doi.org/10.1007/BF03256395
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256395