Abstract
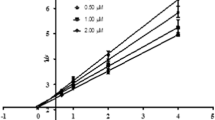
The differences in the inhibition potency of organophosphorus agents are manifestations of differing molecular properties of the inhibitors involved in the interaction with the active site of an enzyme. We studied the inhibition potency (IC50) of phosphoramidates with the general formula [(CH3)2N]P(O)[p-NHC6H4-X]2, where, X = F (1), Cl (2), Br (3), I (4), and [(CH3)2N]P(O)X[p-OC6H4-CH3], where, X = o-NHC6H4-CH3 (5), m-NHC6H4-CH3 (6), p-NHC6H4-CH3 (7), on human acetylcholinesterase (AchE) activity. Inhibition of hAChE was evaluated by a modified Ellman’s method, and spectrophotometric measurements. The ability of the compounds to inhibit AChE was predicted by PASS software (version 1.193). The IC50 values for inhibitors 1–7 were found to be 0.19, 0.35, 0.50, 0.63, 2.70, 2.44 and 1.50 mM, respectively. In addition, logP values, by the experimental and calculated (software) methods, were found to be 1.03, 1.64, 2.17, 2.74, 2.34, 2.28 and 2.23. Finally, parameters of IC50, logP, δ31P (phosphorus chemical shifts), σp (para-substituent constant), σ+ (electron-releasing), steric and topological effects were used for the structure-activity relationships (SAR) studies.
Similar content being viewed by others
Refrences
W. D. Mallender, T. Szegletes, T. L. Rosenberry, Biochem. 39 (2000) 7753.
A. Baldwin, Z. Huang, Y. Jounaidi, D. J. Waxman, Arch. Biochem. Biophys. 409 (2003) 197.
K. Gholivand, A. M. Alizadehgan, F. Mojahed, G. Dehghan, A. Mohamadirad, M. Abdollahi, Z. Naturforsch. 63c (2008) 241.
K. Kamil, B. Jirl, C. Jirl, K. Jirl, Bioorg. Medic. Chem. Lett. 13 (2008) 3545.
A. K. Singh, Com. Biochem. Physico. 123 (1999) 241.
S. Basak, V. Magnuson, G. Niemi, R. Regal, G. Veith, Math. Modeling 8 (1986) 300.
C. Hansch, A. Leo, Exploring QSAR, ACS Professional References Book, Washington DC, 1995.
J. H. Letcher, J. R. Van Wazer, J. Chem. Phys. 44 (1966) 815.
W. Renxiao, F. Ying, L. Luhua, J. Chem. Inf. Comput. Sci. 37 (1997) 615.
S. Ghadimi, V. Khajeh, J. Iran. Chem. Soc. 4 (2007) 325.
S. Ghadimi, S. Mousavi, Z. Javani, J. Enzy. Inhibit. Medic. Chem. 23 (2008) 213.
K. Gholivand, A. Mahmoudkhani, M. Khosravi, Phosphorus Sulfur and Silicon 106 (1995) 173.
K. Gholivand, Z. Shariatinia, A. Tadjarodi, Main. Group. Chem. 4 (2005) 111.
A. Lagunin, A. Stepanchikova, D. Filimonov, V. Poroikov, PASS. Bioinformatics Applications Note 16 (2000) 747.
A. A. Geronikaki, J. C. Dearden, D. Filimonov, I. Galaeva, L. Garibova, T. Gloriozova, V. Kranjneva, A. Lagunin, F.Z. Macaev, G. Molodavkin, V. Poroikov, S. I. Pobrebnoi, F. Shepeli, T. Voronina, M. Tsitlakido, L. Vald, J. Med. Chem. 47 (2004) 2870.
M. Medic-Saric, A. Mornar, T. Badovinac-Crnjevic, I. Jasprica, Croat. Chem. Acta 77 (2004) 367.
N. Hosa, Z. Radic, I. Tsigeling, H. Berman, D. Quinn, P. Taylor, Biochem. 35 (1996) 995.
M. Hoenicka, Anal. Biochem. 25 (1968) 192.
J. Smith, C. Simons, Enzyme and Their Inhibition Drug Development, CRC Press, 2004.
C. Tolman, Chem. Rev. 77 (1977) 313.
E. Fluck, G. Heckmann, In Phosphorus-31 NMR Spectroscopy in Stereochemical Analysis, VCH, Weinheim, 1987.
E. Breitmair, W. Voelter, 13C NMR Spectroscopy, 3rd ed., VCH, Weinheim, 1990.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghadimi, S., Ebrahimi-Valmoozi, A.A. Lipophilicity, electronic, steric and topological effects of some phosphoramidates on acethylcholinesterase inhibitory property. JICS 6, 838–848 (2009). https://doi.org/10.1007/BF03246178
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246178