Abstract
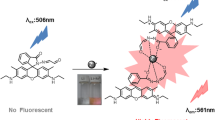
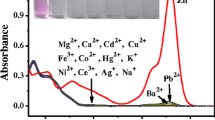
We have developed and characterized a new Rhodamine-based Fe3+ selective fluorescent turn-on chemosensor. A new Rhodamine-based fluorescent sensor Rh1 was synthesized by condensation and reduction of compound 1 with Salicylaldehyde. Fluorescent sensor Rh1 exhibited a good selectivity and little interference toward Fe3+ over other metal ions in acetonitrile. Fluorescent sensor Rh1 was colorless and non-fluorescent in the absence of Fe3+, pink color and strong fluorescence was observed after addition of Fe3+. Since the fluorescent sensor Rh1 undergoes 1,000 fold increase in fluorescence intensity along with color change upon binding with Fe3+, implying possible applications in a variety of area such as environmental monitoring and diag]nostic analysis.
Similar content being viewed by others
References
Brugnara, C. Iron deficiency and erythropoiesis: new diag]nostic approaches.Clin. Chem. 49, 1573–1578 (2003).
Beard, J. Iron deficiency alters brain development and functioning.J. Nutr. 133, 1468S-1472S (2003).
Egan, T.J.et al.Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of beta-hematin formation, and antiplasmodial activity.J. Med. Chem. 43, 283–291 (2000).
http://www.epa.gov/ogwdw000/consumer/2ndstandar ds.html.
Gunnlaugsson, T., Leonard, J. P. & Murray, N. S. Highly selective colorimetric naked-eye Cu(II) detection using an azobenzene chemosensor.Org. Lett. 6, 1557–1560 (2004).
Royzen, M., Dai, Z. & Canary, J. W. Ratiometric displacement approach to Cu(II) sensing by fluorescence.J. Am. Chem. Soc. 127, 1612–1613 (2005).
Krämer, R. Fluorescent chemosensors for Cu2+ ions: fast, selective, and highly sensitive.Angew. Chem., Int. Ed. 37, 772–773 (1998).
Jiang, P. J. & Guo, Z. J. Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors.Coord. Chem. Rev. 248, 205–229 (2004).
Zeng, L., Miller, E. W., Pralle, A., Isacoff, E. Y. & Chang, C. J. A selective turn-on fluorescent sensor for imag]ing copper in living cells.J. Am. Chem. Soc. 128, 10–11 (2006).
Xiang, Y. & Tong, A. A new rhodamine-based chemosensor exhibiting selective FeIII-smplified fluorescence.Org. Lett. 8, 1549–1552 (2006).
Bricks, J. L.et al.On the development of sensor molecules that display FeIII-amplified fluorescence.J. Am. Chem. Soc. 127, 13522–13529 (2005).
Zhang, M.et al.A selective turn-on fluorescent sensor for FeIII and application to bioimag]ing.Tetrahedron Lett. 48, 3709–3712 (2007).
Bricks, J. L.et al.On the development of sensor molecules that display FeIII-amplified fluorescence.J. Am. Chem. Soc. 127, 13522–13529 (2005).
Tumambac, G. E., Rosencrance, C. M. & Wolf, C. Selective metal ion recognition using a fluorescent 1,8-diquinolylnaphthalene-derived sensor in aqueous solution.Tetrahedron 60, 11293–11297 (2004).
Ma, L., Luo, W., Quinn, P. J., Liu, Z. & Hider, R. C. Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors.J. Med. Chem. 47, 6349–6362 (2004).
Weizman, H.et al.Fluorescently-labeled ferrichrome analogs as probes for receptor-mediated, microbial iron uptake.J. Am. Chem. Soc. 118, 12368–12375 (1996).
Xiang, Y., Tong, A.-J., Jin, P.-Y. & Ju, Y. New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity.Org. Lett. 8, 2863–2866 (2006).
Kim, H. N., Lee, M. H., Kim, H. J. & Kim, J. S. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions.Chem. Soc. Rev. 37, 1465–1472 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, T., Yang, S.I. A new Rhodamine-based turn-on fluorescent chemosensor for Fe3+ . Toxicol. Environ. Health. Sci. 2, 73–77 (2010). https://doi.org/10.1007/BF03216515
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03216515