Summary
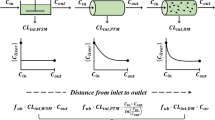
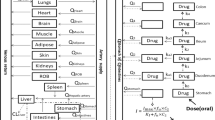
The bioavailability of a new retard tablet formulation of verapamil was investigated in a randomized cross-over bioequivalence study on 12 healthy subjects. The drug was given in the form of a single 240-mg oral dose of a new retard tablet formulation, or as a standard retard tablet at the same dose to all subjects, followed by a single intravenous (i.v.) dose of 5 mg to 8 of the 12 subjects. Plasma verapamil concentrations were determined by a high performance liquid chromatography (HPLC) method. The bioavailability of the new peroral retard formulation was (20.00±4.30)% and was in reasonable agreement with that determined for the already registered verapamil retard formulation, i.e. (19.46±4.02)%, thereby indicating bioequivalence. For the prediction of systemic availability and estimation of the first-pass metabolism, only based on the data for peroral plasma levels, a hepatic blood flow rate limited model was used. In our experience, this model has been found to be extremely useful in providing reasonable estimates of verapamil first-pass effect.
Similar content being viewed by others
References
Gibakii M., Boyes R.N., Feldman S. (1971): Influence of first-pass effect on availability of drugs on oral administration. J. Pharm. Sci., 60, 1338–1340.
Perrier D., Gibaldi M., Boyes R.N. (1973): Prediction of systemic availability from plasma-level data after oral drug administration. J. Pharm. Pharmacol, 25, 256–257.
Gibaldi M., Perrier D. (1975): Pharmacokinetics. New York, Marcel Dekker.
Vaughan D.P. (1975): Estimation of biological availability after drug administration when the drug is eliminated by urinary excretion and metabolism. J. Pharm. Pharmacol., 27, 458–461.
McLean A.J., McNamara P.J., du Souich P., Gibaldi M., Lalka D. (1978): Food, splanchnic blood flow, and bioavailability of drugs subject to first-pass metabolism. Clin. Pharmacol. Ther., 24, 5–10.
Popović J. (1985): Estimation of the first-pass metabolism of a drug during multiple oral dosage. Period. Biol., 87, 290–292.
Popović J. (1985): Influence of first-pass effect on availability of drugs with simultaneous biotransformation in the liver and first-order elimination through kidneys. Iugoslav. Physiol. Pharmacol. Acta, 21 (Suppl. 3), 289–290.
Popović J. (1986): Dosage regimen calculations for drugs with first-order absorption, non-linear first-pass metabolism and parallel non-linear and first-order elimination. Period. Biol., 88, 183–184.
Popović J. (1987): Relationship between the steady-state serum level and the dose of drugs with first-pass and parallel Michaelis-Menten and first-order elimination. Acta Pharm. Jugosl., 37, 313–317.
Popović J., Mikov M., Jakovljević V. (1992): Pharmacokinetics of ticlopidine derived from a new tablet formulation. Acta Biol. Med. Exp., 17, 49–52.
Popović J., Mikov M., Jakovljević V. (1993): Pharmacokinetics and systemic availability of a new metoprolol retard formulation. Eur. J. Drug Metab. Pharmacokinet., Special Issue.
Popović J., Mikov M., Jakovljević V. (1994): Pharmacokinetic analysis of a new acenocoumarol tablet formulation during a bioequivalence study. Eur. J. Drug Metab. Pharmacokinet., 19, 85–89.
Popović J.. Mikov M., Jakovljević V. (1995): Pharmacokinetics of carbamazepine derived from a new tablet formulation, Eur. J. Drug Metab. Pharmacokinet., 20, 297–300.
Popović J. (2004): Classical Michaelis-Menten and system theory approach to modeling metabolite formation kinetics. Eur. J. Drug Metab. Pharmacokinet., 29, 205–214.
Schomerus M., Spiegelhalder B., Stieren B., Eichelbaum M. (1976): Physiological disposition of verapamil in man. Cardiovasc. Res., 10, 605–612.
Singh B.N., Ellrodt G., Peter T.C. (1987): Verapamil: A review of its pharmacological properties and therapeutic use. Drugs, 15, 169–197.
Freedman S.B., Richmond D.R., Ashley J.J., Kelly D.T. (1981): Verapamil kinetics in normal subjects and patients with coronary artery spasm. Clin. Pharmacol. Ther., 30, 644–652.
Somogyi A., Albrecht M., Kleims G., Schafer K., Eichelbaum M. (1981): Pharmacokinetics, bioavailability and ECG response of verapamil in patients with liver cirrhosis. Br. J. Clin. Pharmacol., 12, 51–60.
Johnston A., Burgess CD., Hamer J. (1981): Systemic availability of oral verapamil and effect on PR interval in man. Br. J. Clin. Pharmacol., 12, 397–400.
Eichelbaum M., Somogyi A., von Unruh G.E., Dengler H.J. (1981): Simultaneous determination of the intravenous and oral pharmacokinetic parameters of D, L-verapamil using stable isotope-labelled verapamil. Eur. J. Clin. Pharmacol., 19, 133–137.
Kates R.E., Keefe D.L., Schwartz J., Harapat S., Kirsten E.B., Harrison D.C. (1981): Verapamil disposition kinetics in chronic atrial fibrillation. Clin. Pharmacol. Ther., 30, 44–51.
Woodcock B.G., Rietbrock I., Vohringer H.F., Rietbrock N. (1981): Verapamil disposition in liver disease and intensivecare patients: kinetics, clearance, and apparent blood flow relationships. Clin. Pharmacol. Ther., 29, 27–34.
McAllister R.G., Kirsten E.B. (1982): The pharmacology of verapamil: IV-kinetic and dynamic effects after single intrave-nous and oral doses. Clin. Pharmacol. Ther., 31, 418–426.
Kates R.E. (1983): Calcium antagonists. Pharmacokinetic properties. Drugs, 25, 113–124.
Hamann SR., Blouin R.A., McAllister R.G. (1984): Clinical pharmacokinetics of verapamil. Clin. Pharmacokinet., 9, 26–41.
Vicek J., Macek K., Hulek P., Bratova M., Fendrich Z. (1995): Pharmacokinetic parameters of verapamil and its active metabolite norverapamil in patients with hepatopathy. Arzneimforsch., 45, 146–149.
Krecic-Shepard M.E., Bamas CR., Slimko J., Jones M.P., Schwartz J.B. (2000): Gender-specific effects on verapamil pharmacokinetics and pharmacodynamics in humans. J. Clin. Pharmacol., 40, 219–230.
Vogelgesang B., Echizen H., Schmidt E., Eichelbaum M. (2004): Stereoselective first-pass metabolism of highly cleared drugs: studies of the bioavailability of L- and D-verapamil examined with a stable isotope technique. 1984. Br. J. Clin. Pharmacol., 58, pp. S796–803; discussion S804–6.
Sica D.A. (2005): Calcium channel blocker class heterogeneity: select aspects of pharmacokunetics and pharmacodynamics. J. Clin. Hypertens., 7, 21–26.
Kovarik J.M. (2005): Pharmacokinetic interaction between verapamil and everolimus in healthy subjects. Br. J. Clin. Pharmacol., 434–437.
Harapat S.R., Kates R.E. (1979): Rapid high-pressure liquid chromatographic analysis of verapamil in blood and plasma. J. Chromatogr., 170, 385–390.
Yeh C.K., Kwan C.K. (1978): A comparison of numerical integrating algorithms by trapezoidal, Lagrange and spline approximation. J. Pharmacokinet. Biopharm., 6, 79–98.
Popović J., Popović V. (1985): Cubic spline functions in pharmacokinetic data analysis. Period. Biol., 87, 293–296.
Popović J., Popović V. (1993): Analysis of toxicokinetic data by means of spline functions. Arch. Toxicol. Kinet. Xenobiot. Metab., 1, 79–93.
Popović J. (1997): Polynomials vs cubic spline functions for model independent deconvolution calculations of absorption rate. Eur. J. Clin. Pharmacol., 52 (Suppl.), 446.
Popović J. (1998): Cubic spline function and polynomials for deconvolution calculations of absorption rate — numerical evaluation. Arch. Toxicol. Kinet. Xenobiot. Metab., 6, 99–107.
Popović J. (1998): Cubic spline functions and polynomials for calculation of absorption rate. Eur. J. Drug Metab. Pharmacokinet., 23, 469–473.
Bauer L.A., Horn J.R., Scot Maxon M., Easterling T.R., Shen D.D., Strandness D.E. (2000): Effect of metoprolol and verapamil administered separately and concurrently after single doses on liver blood flow and drug disposition. J. Clin. Pharmacol., 40, 533–543.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Popović, J. Validation of the hepatic blood flow rate model for verapamil first-pass metabolism. Eur. J. Drug Metabol. Pharmacokinet. 32, 13–19 (2007). https://doi.org/10.1007/BF03190985
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190985