Summary
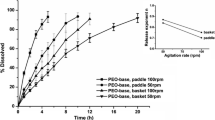
The purpose of this study was to investigate the possibility of developing different levels of in vitro-in vivo correlation for immediate-release paracetamol tablets using in vitro dissolution data obtained under various experimental conditions. The influence of agitation intensity and pH value of the dissolution media was investigated. The level B approach, using statistical moment analysis led to poor correlation results. The results obtained by numerical deconvolution in order to study level A correlation indicated that good correlation should be sought with moderate levels of agitation (beyond 50 rpm in rotating basket apparatus). Results obtained by numerical convolution showed the highest level of correlation, level A, with one-to-one relationship between observed and predicted in vivo profiles.
Similar content being viewed by others
References
Katori N., Aoyagi N., Terao T. (1995): Estimation of agitation intensity in the GI tract in humans and dogs based on in vitro in vivo correlation. Pharm. Res., 12, 237–243.
Ishii K., Saitou Y., Yamada R., Itai S., Nemoto M. (1996): Novel approach for determination of correlation between in vivo and in vitro dissolution using the optimization technique. Chem. Pharm. Bull., 44, 1550–1555.
Mattok L.G., McGilveray I.J., Mainville A.C. (1971): Acetaminophen III: dissolution studies of commercial tablets of acetaminophen and comparison with in vivo absorption parameters. J. Pharm. Sci., 60, 561–564.
Sotiropoulus B.J., Deutsch T., Plakogiannis M.F. (1981): Comparative bioavailability of three commercial acetaminophen tablets. J. Pharm. Sci., 70, 422–425.
Forrest J.A.H., Clements J.A., Prescott L.F. (1982): Clinical pharmacokinetics of paracetamol. Clin. Pharmacokinet., 7, 93–107.
Sartorius dissolution simulator, Instruction manual. Sartorius GmbH, Götingen, Germany
Brockmeier D. (1986). In vitro/in vivo correlation of dissolution using moments of dissolution and transit times. Acta Pharm. Technol., 32, 164–174.
Radovanović J., Durić Z., Jovanović M., Ibrić S., Nikolić L. (1997): The influence of pH and agitation intensity on drug release from tablets evaluated by means of factorial design, Presented at 16th Pharmaceutical Technology Conference, Athens, Greece, April, 15–17.
Cardot J.M., Beyssac E. (1993): In vitro/in vivo correlations: scientific implications and standardisation. Eur. J. Drug Metab. Pharmacokinet., 18, 113–120.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Radovanović, J., Durić, Z., Jovanović, M. et al. An attempt to establish an in vitro-in vivo correlation: case of paracetamol immediate-release tablets. European Journal of Drug Metabolism and Pharmacokinetics 23, 33–40 (1998). https://doi.org/10.1007/BF03189824
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189824